Is the Pfizer/BioNTech a DNA Vaccine?
Or is the DNA required as an adjuvant? Speculation only
So I was listening to a talk by Dr Ulrike Kammemer, a molecular biologist, who has worked with Kevin McKernan and published on the residual DNA and the SV40 promoter in her excellent paper.
Here is the video
Prof. Dr. Ulrike Kämmerer – genetisch verändert durch C-Injektion?
And although in German, you can auto translate to English subtitles. At about 31:00 brings up this paper.
1. SV40-72bp Element in DNA Vaccines
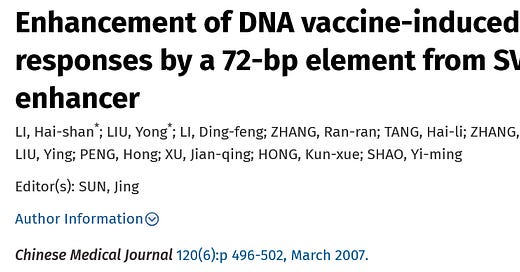
Well, well, well. This was a Chinese study for an proposed HIV DNA vaccine. The DNA plasmid vaccine was compared to a viral vector vaccine (like the AZ vaccine) using vaccinia (smallpox) as their vector.
Here they made 2 DNA plasmids, one with and one without the SV40 72bp tandem repeat which they produced in E coli. The plasmids were making gag protein of the HIV-virus. So p-gag has no SV40 and pSV-gag has the SV40 72bp element. Note it was only a single copy of the 72bp element, not the entire enhancer/promoter/ori found in the Pfizer vaccine which is >300bp and the 72bp element is a tandem REPEAT in the mRNA vaccine. I can’t tell from the paper if they used LNPs or electroporation with the DNA plasmid vaccine. Maybe it was naked plasmids?
Here is what they showed in HEK cells for expression of the gag protein on a Western blot. They used lipofectamine to transfect the HEK cells with the DNA plasmids for these Western blots.
A1 is the DNA plasmid with the SV40 72bp element; A2 is the DNA vaccine without the SV40, A3 and A4 are control plasmids without the gag protein. B1 is the vaccinia vector vaccine. B2 is a control vaccinia vector vaccine.
They conclude:
Therefore, more expression of gag protein occurs because the SV40 sequences allow nuclear targeting, even in non-dividing cells. Ahem.
2. Plasmid DNA lengths in the Pfizer/BioNTech Vaccine
OK. So is the Pfizer vaccine a DNA vaccine? In order for the current mRNA vaccine to work as a DNA vaccine, this would require that the plasmid DNA in the vials is coding for the spike protein. In this case that would be a large chunk of linear plasmid DNA of about 3,800 bp. Mind you, Kevin McKernan did find a piece of DNA almost that big when examined a vial using Nanopore sequencing, as seen in our preprint. However, most DNA fragments were smaller.
Is is possible that if more vials were tested for the size of the DNA fragments that we would see pieces big enough to code for the entire spike protein? Or was this finding a fluke? Is it a random finding that every say 1000 vials have residual DNA that could possibly code for the spike protein?
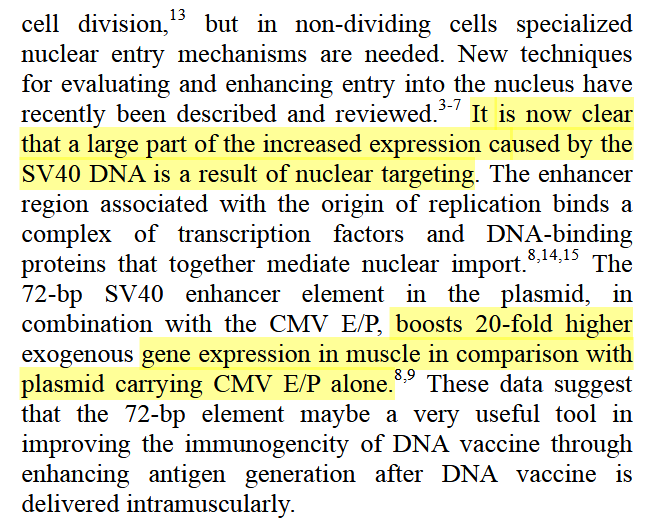
3. Health Canada Asks for the Size Distribution of the DNA Fragments
If we remember, after Health Canada figured out there was indeed lots of DNA and the SV40 promoter/enhancer, they asked Pfizer for an explanation and for the DNA size distribution in point 3 below.
Please see my earlier substack on this.
However, the answer back from Pfizer has all been redacted, though
has been trying very hard to get the Health Canada, through Canada’s Information Commissioner, to cough up the data. The initial report is here.Typical.
4. That Strange Process 1 Lot RNA-RF200321-06
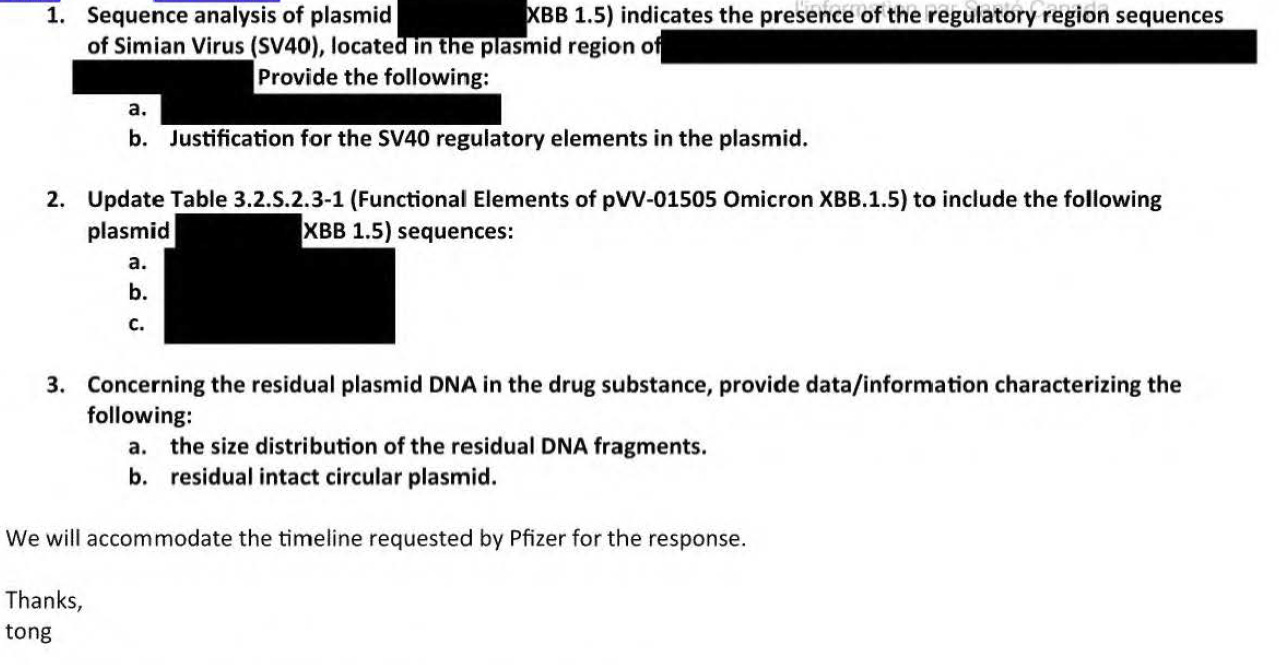
I have always been bothered by the very first lot of the modRNA BNT162b2 which was made on March 27, 2020 as shown below. It was called an engineering lot, and it was a non-clinical and toxicology lot. This lot was never used in patients. Note this is JUST the modRNA.
Now this lot has 815.3 ng DNA per mg RNA. This is about 24.5ng/dose. BioNTech helpfully adds that Oh well, our DNase didn’t work. Even with this very first lot, they had problems with their DNase enzyme. Why is that? However, it was March 2020. Was there not time to make another engineering lot that had less DNA in it?
Keep in mind, they used PCR to quantify the amount of DNA. There was no fluorometry used to quantify the DNA in any lot reviewed by the EMA in the rolling review.
The 4 lots starting with R are Process 1 lots. As you can see, Process 1 lots had varying amounts of residual DNA. It is a FALLACY that Process 1 lots did not have residual DNA in them. They did. Levels ranged from very low (1ngDNA/mgRNA = 0.03ng/dose) to just under 200ng/DNA/mgRNA (6ng/dose). It is not plasmid DNA however, and no SV40 sequences.
The process 2 lots starting with 20Y513C had more variability but ranged from 10ngDNA/mgRNA (0.3ng/dose) to 211ngDNA/mgRNA (6.3ng/dose).
So where was this lot with huge amounts of residual DNA used? None of the subsequent lots had residual DNA quantities as high as that found with this first lot. And it was made in Mainz and not in Idar-Oberstein where all the other Process 1 mRNA lots were made. (Note the Process 2 lots using the Plasmid DNA were made in Andover Mass in the US).
This special lot was used in the animal toxicity studies and the animal immunological studies. In particular, this lot was used in
Immunogenicity, transmission, study in non-human primates
This was the most important efficacy and safety study done in animals and was published as a preprint December 2020 and then as a full article February 2021 in Nature.
If however, you read the submission of this study to the FDA, study VR-VTR-10671 it can be found at this link on the Public Health and Medical Professionals (PHMP) for Transparency site as a result of Aaron Siri’s legal action on Pfizer.
So now we look at what is called the TEST ARTICLE in this document to see what batch they used of the mRNA which is on Page 8. Here you see Lot Number CoVVAC/270320. They used these types of numbering for the completed product, ie the mRNA/LNP vaccine. See here they said provided by BioNTech in Mainz Germany. Already a clue.
OK, now I go to the Rolling Review from the EMA leak/hack and try to find this Lot number CoVVAC/270320 and I get this
As you can see, they used the RF200321-06 mRNA batch first made 27 Mar 2020 to make the vaccine lot CoVVAC/270320 the final vaccine formulation. So this lot had 24ng/dose of DNA by PCR.
It is best to read the study on the PHMP site since it specifically just BNT162b2 in macaques using this lot. The published Nature study includes a whole bunch of other studies. Here are some results in macaques. Not much difference between the 30ug and 100ug dose though.
I have gone through the PHMP site for all the animal toxicology and immunology studies and this mRNA lot RF200321-06 used to make batch CoVVAC/270320 (ie Test Item) with excessive amounts of in DNA, was also used in the following studies.
R-20-0085 Immunogenicity in mice
-performed March 31st to September 17, 2020 (only days after it is made)
R-20-0112 Immunogenicity in mice
-May 5-June 4, 2020
R-20-0211 in vitro expression, western blots and potency testing
-June 22-Aug 14, 2020
20GR142 toxicity study in rats
-June 23-Aug 13, 2020
20256434 the DART study in rats (development and reproducibility)
-Sept 27-Dec 10, 2020
This mRNA lot full of DNA, in which the DNase 1 has failed. WHY WAS THIS LOT USED? Could it be that it contain the full DNA sequence for the spike protein and was in fact a DNA vaccine first and an mRNA vaccine second? However, this lot would not contain the SV40 sequences I don’t believe since the DNA template was PCR amplified so no promoters are needed. Please correct me if I am wrong. We do not have the size distribution for this lot.
Was this lot used because it gave less toxicity results and higher immunogenicity than a pure mRNA shot would? Can anyone help?
Or maybe because the DNA acts like an adjuvant and they wanted the best possible immunological results?
5. Results from the Slovak Republic and Ireland
Recently the Slovak government made an announcement regarding residual DNA in 24 lots of both Moderna and Pfizer. They used PCR, so it is comparable to the results from Pfizers submission to the regulators as presented here on X.
Here is the update to the google sheet maintained by Kenji Fujikawa.
And recent results on the most recent Irish vials by David Speicher delineated on his substack.
So large amounts of DNA PLUS the SV40 sequences remain even in 2025. WHY? I do wish we had more data on the size distribution however since the DNA could be broken up and in sizes too small to code for the entire spike protein.
But WHY does the Pfizer vaccine continue to have such large amounts of DNA and still have the SV40 sequences after more than 4 yrs of production? It doesn’t make sense.
6. DNA as an adjuvant?
Maybe there remains a large amount of DNA in the Pfizer vaccines not because it has intact plasmids and acts like a DNA vaccine, but because large amounts of plasmid DNA act as an adjuvant?
In this recent paper they review all the types of adjuvants used in current vaccines. Interestingly, they place the LNPs as the adjuvant for the mRNA vaccines. More later.
Here is what they say about unmethylated CpG motifs, CpG 1018 and CpG 7909, which are two TLR9 agonists utilized in previously licensed vaccines.
CpG motifs, comprised of a central unmethylated CG dinucleotide plus flanking regions, are derived from synthetic oligodeoxynucleotides (ODN) which mimic bacterial DNA.
Bacterial DNA represents a potent pathogen-associated molecular
pattern (PAMP), which activates the innate immune system.
So these are synthetic DNA sequences mimicking bacterial DNA. A HAH!!! Oh, so these motifs are not very big I would presume. Maybe 15-30 nucleotides big? So how many would you need of these small pieces of plasmid DNA to act as an adjuvant?
CpG 1018 was first utilized in a licensed vaccine in 2017 for the Hep-B vaccine, Heplisav-B; CpG 7909 was then utilized after this in July 2023 in the licensed anthrax vaccine, Cyfendus together with an aluminum adjuvant. Yikes.
So how much is required to be an adjuvant in ng?
Grok tells me
CpG 1018, a synthetic TLR9 agonist, is used as an adjuvant in vaccines like Heplisav-B (3000 μg per dose) and some SARS-CoV-2 candidates, such as Medigen’s MVC-COV1901, where it’s combined with alum and a recombinant spike protein (750 μg per dose in Phase 2 trials).
Whew. That’s a lot. But these are classic antigen vaccines. What about inside LNPs? How much would you need?
Grok makes a guess
…studies on cancer vaccines have tested LNPs co-delivering mRNA and CpG ODNs to enhance immune responses. In a 2023 study, LNPs encapsulating tumor antigen mRNA and CpG ODNs (not specifically CpG 1018) showed improved CD8+ T-cell responses in melanoma models, with CpG doses around 5–10 μg in mice (scaled to nanograms, 5,000–10,000 ng). Another study on self-amplifying mRNA (saRNA) vaccines for SARS-CoV-2 tested LNPs with CpG ODNs, finding that 10 μg (10,000 ng) per dose in mice boosted Th1 responses synergistically with the LNP’s intrinsic adjuvanticity. These amounts are far lower than the 750,000–3,000,000 ng seen in human subunit vaccines, reflecting differences in delivery and scale.
Well, those are number getting closer to what we find with fluorometry where we are measuring those small bits of DNA sequences. Still based on the evidence thus far, the most that has been measured is about 5000ng or about 5ug. Not quite enough as what has been used as an adjuvant but getting there. Maybe we aren’t measuring everything though?
OK I’m going to digress here. LNPs as adjuvants?
What about the LNPs? Are they considered adjuvants? This article states that in the current clinical trial landscape, there are 75 unique vaccine formulations using nanoparticle-based adjuvants with a total of 98 trials consisting of Matrix-M, gold nanoparticles, lipid nanoparticles, and other variations of lipid carriers. Holy Toledo.
IN WHICH I RANT
Of course the LNPs are adjuvants and should have been assessed by the regulators as such. AT LEAST. Instead they were novel excipients which meant they had no pharmacological actions on their own. So no review. And this sets a precedent for further LNP formulations. I’m beginning to think this is as bad from a regulatory point of view as the discovery of the SV40 sequences. Look at the number of clinical trials using nanoparticles as adjuvants (not all are true LNPs though).
Summary and Questions
The SV40 promoter/enhancer has been studied in DNA vaccines to promote more antigen production since the SV40 brings DNA into the nucleus in non-dividing cells.
Can the SV40 promoter/enhancer be used for naked DNA vaccines (without LNPs) to stimulate an immune response. Can someone comment? Possible? How much would you need?
With the large amounts of residual DNA could the Pfizer vaccine represent a DNA vaccine?
evidence for large amounts of plasmid DNA constructs of 3.5kb or more are lacking.
we have no evidence thus far on the average size distribution of DNA in the vials despite Health Canada asking for this info, it remain redacted.
more research on size distribution of the DNA fragments maybe useful
there may be variations between lots requiring a large sampling size
Maybe the DNA is left in the Pfizer vaccine because it acts as an adjuvant?
this maybe supported by the strange process 1 lot with about 24ng/dose of residual DNA by PCR which was used for the animal toxicity and immunogenicity trials
speculation only: the large amount of DNA in this process 1 lot was deliberately left in there so it could stimulate a Th1-biased immune response, which is characterized by strong innate immunity and the promotion of cellular immunity alongside antibody production? Otherwise if not, there would not be an adequate T-cell response? That this needed to be shown in the animal studies in particular or the vaccine would be a dud? Is this even more fraud?
Remember that the clinical trials in humans were started BEFORE all the animal immunogenicity and toxicology trials were completed. I guess I need to look into whether the pivotal Polack trial looked to see if the human subjects had a strong Th1-based immune response. But I am not strong in immunology. Sorry. Can someone help?
What about Moderna? Are they using DNA fragments as an adjuvant? I know Moderna’s product had lots of dsRNA in it compared to Pfizer and THAT is certainly acting as an adjuvant.
Were the SV40 sequences used as a promoter in the plasmid and Pfizer told the regulators it acted as an adjuvant in the vaccine to escape censure?
does the SV40 sequences contain unmethylated CpG motifs as present in the adjuvant in the hepatitis B vaccine?
Holy Toledo. Grok tells me
The promoter/enhancer region specifically contains multiple CpG dinucleotides. For example, within the 72-bp enhancer repeats and adjacent sequences, unmethylated CpG sites are present.
Is this true? Anyone? Maybe they are not optimized for immune stimulation.
If the residual DNA and/or the SV40 enhancer are acting as adjuvants, is this the reason why high levels of DNA and the SV40 sequences remains? Is this the reason why the regulators have gone quiet and have not insisted on withdrawal or a change in the label? But what about the variability in residual DNA that remains? Why has that continued?
And if true, what does this tell you about the effectiveness of an mRNA vaccine?
For background I speculated that the Pfizer vaccine was using DNA as an adjuvant and Moderna was using dsRNA as an adjuvant in my very first substack.
This does not negate other issues about the mRNA, codon optimization, template switching, reverse transcription etc. Or about the risk of cancer and other effects of plasmid DNA in the cytosol, or in the nucleus.
OK everyone, these are just speculations and questions. There is no doubt that it is hard to get rid of all the residual DNA in these products. And hard to measure exactly how much is in there. But I see no real improvement over time. And continued variability.
Maybe I am off my rocker. Please correct/educate me in the comments. Thanks.
And pray the rosary. Always



















Forget nanograms Synthetic GMO Plasmid DNA in mRNA Jabs, the E. Coli Genomic DNA has a limit of 100 picogram per dose imposed by the FDA because it is considered extremely dangerous. That is too high.
https://geoffpain.substack.com/p/bacterial-dna-a-major-worry-in-jabs
Thanks for your work. I don't have much scientific training, but I make an effort by reading articles like yours.
When I do, my sense is always strengthened that this technology makes informed consent impossible.