It's the double stranded RNA impurities that is the issue
Both Pharma and Regulatory authorities don't seem to care about DNA contamination
Read the Business News/Trade Publications to know what Pharma is doing
I learned to read the Business News to find out what the next marketing campaign Pharma was thinking about with their new drug, who they think their competitors are and how they can make more money on a new scientific advance.
Reading papers on manufacturing and business news on mRNA, no one is interested AT ALL in DNA contamination or that it is even a THING.
In this trade editorial of a company which supply equipment to biotechnology companies is a very good review on mRNA technology and the quality control needed.
mRNA Manufacturing - BioCompare
Outline of mRNA vaccine manufacturing
The manufacture of an mRNA vaccine consists of the following basic steps or variations thereof:
Plasmid/template creation and maintenance
Plasmid/template isolation, purification, and preparation for transcription into mRNA
mRNA transcription
mRNA post-transcriptional modification, i.e., 5’ capping and 3’ poly-A tail addition
mRNA purification
Formulation with cationic lipids
Plasmid DNA Contamination
They correctly state it all starts with a good quality plasmid DNA and a master cell bank. BUT
During the purification process, little, if any, source plasmid should remain as the DNA is digested with DNase post-transcription; hence, plasmid persistence studies will not be necessary for mRNA vaccines and most likely will not be covered by a future mRNA vaccine-dedicated guidance.
What??!! Do they know something we don’t? Plasmid DNA vaccines are covered by FDA Guidelines from 2007, which considered plasmid DNA integration but this was considered not a real risk since:
intramuscular studies in mice or guinea pigs using four different DNA vaccine plasmids demonstrated that there was no evidence of integration using an assay with a sensitivity of about one plasmid copy/microgram of DNA, which would at least three orders of magnitude below the spontaneous mutation frequency (Ledwith BJ et al, 2000).
Here is the abstract for the Ledwith paper
So although residual DNA, enzymes, solvents, truncated mRNA etc etc should be identified and mitigated, THEY DON’T REALLY NEED TO WORRY TOO MUCH ABOUT DNA PLASMID CONTAMINATION. See? Already proven that integration is not a risk. Just make sure you are below the magic 10ng/dose and <200 base pair fragments and you are good with the FDA. No wonder we are getting crickets from the regulators.
However, mRNA vaccine technology has not considered the impact of
transfection of the DNA as opposed to naked DNA in blood vessels as with DNA vaccines
the amount and potential immunostimulatory and other effects of lots of small fragments of foreign DNA
the SV40 promoter
But from a Pharma point of view, they don’t need to worry about it until the regulators tell they them they need to.
The talk is all about dsRNA
HOWEVER, everything I read is looking at double stranded RNA. But it isn’t being measured well. This may be more important than the plasmid DNA, a least for side effects.
The introduction of impurities typically occurs during the capping stage of mRNA vaccine production, where a cap structure is added to enhance mRNA translation and provide protection and stability. Ideally, vaccines should consist of 100% pure single-stranded mRNA since caps can only be added to single-stranded molecules. Unfortunately, unwanted double-stranded mRNAs may be present, compromising purity
How does dsRNA occur? It happens when you perform IVT with the T7 promoter, and I presume the SVC40 promoter as well. Plus there is another mechanism in which dsRNA is produced that is independent of promoter.
In the first mechanism, the RNA transcript synthesized by the T7 RNA polymerase (RNAP) serves as a template for the RNA-dependent RNA polymerase activity of the T7 RNAP in subsequent rounds of transcription. If the 3’-end of the runoff transcript has sufficient complementarity (in cis), it will fold back and result in extension of the runoff transcript. In the second mechanism, the formation of dsRNA byproducts results from the RNAP using the nontemple strand, resulting in an RNA molecule that is complementary to the runoff product but synthesized in a promoter-independent manner. Because the size of the antisense molecule will be similar to the size of the runoff product, it cannot be distinguished by denaturing gel electrophoresis; rather, analysis of the dsRNA byproducts formed because of the presence of an antisense RNA molecule will require native conditions.
So when you measure how much mRNA you have, some of it could be dsRNA and YOU WONT KNOW IT IS THERE necessarily using the normal analytical methods for measuring the quantity of mRNA for use in the vaccines.
Measuring Impurities and dsRNA
This company wants to sell devices and analytical tests but so these all these methods for measuring dsRNA is quite impressive. For Pfizer and Moderna? They are just using immunoblot techniques at present (though they may have expanded and are using other methodologies by now, I hope). Just look at the PURITY of the mRNA for now.
multiple tests for dsRNA
immunoblotting which is currently being done
electrophoresis, not one but TWO separate types
mass spec
capping analysis (currently a calculated number and not directly measured)
poly A tail analysis (using ddPCR and HPLC and not mass spec as suggested here, heterogeneity of poly A tails can be an issue)
Wow. That is a lot of work.
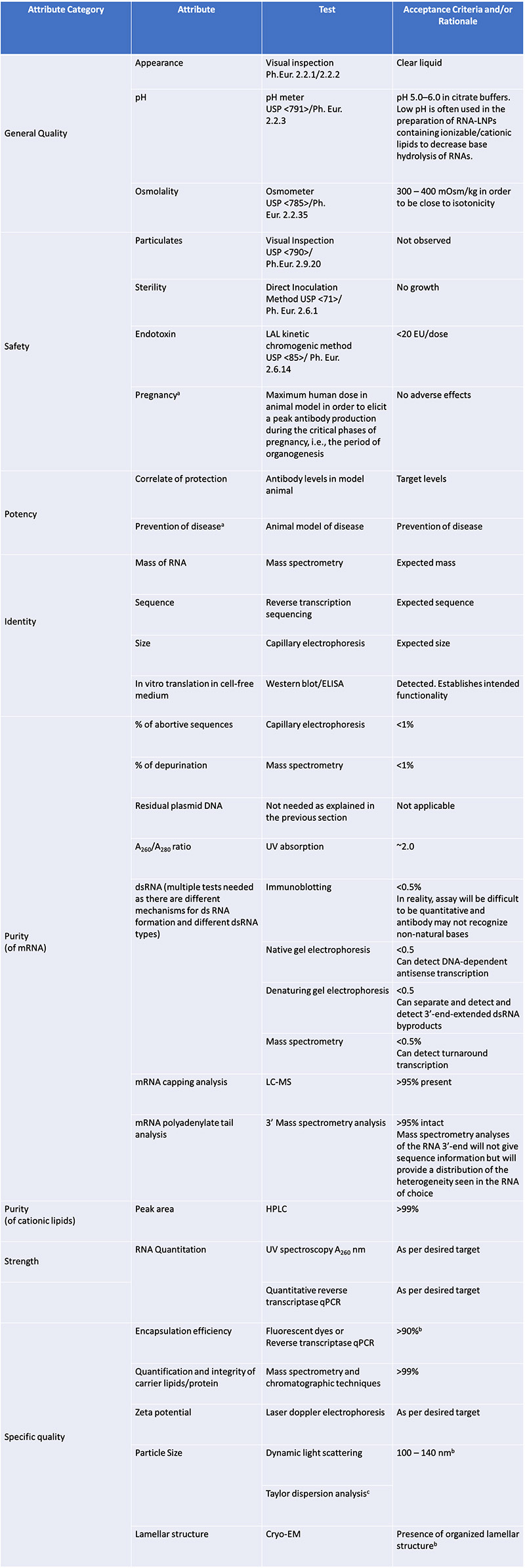
Table 2: Suggested mRNA vaccine drug product quality attributes
How to get rid of dsRNA
You KNOW dsRNA is a problem when you have 2 major new paper inventing new techniques in the 1.5yrs that looks at limiting the amount made at the source, and to avoid costly and time consuming measuring and purification for dsRNA
invent a new T7polymerase like Moderna did
An engineered T7 RNA polymerase that produces mRNA free of immunostimulatory byproducts
invent a new 5’ cap like these Japanese researchers did
PureCap technique, for producing mRNA vaccines with exceptional purity and high activity
So with all the focus on the plasmid DNA contaminating the vials, the manufacturers themselves are focusing on getting rid of dsRNA instead.
WHY?
This signals to me that the DNA contamination is known and is either not a problem for the regulatory authorities or does not contribute to side effects. It is the dsRNA which is causing much of the adverse events and if Moderna or another pharma company can market their new product as free from risks of myocarditis, clots etc, these new discoveries will pave the way.
So I am not sure that contamination with dsDNA in the vaccines will move the needle with respect to the regulatory authorities OR Pharma with respect to these jabs. Au contraire, I think the marketing will be on improving the impurities and see? all good now. No myocarditis or pericarditis, no clots, no cancer yada yada.
I would expect this even if these were normal vaccines and these were normal times.
NOW? Even more so.
Time will tell.



I read this weekly. Highlighted this article for you.
https://www.drovers.com/news/education/livestock-and-mrna-vaccines-what-you-need-know
You might like
https://geoffpain.substack.com/p/septic-shock-induced-by-plasmid-dsdna