Lipid Adducts
A contaminant no one knows about but may be another risk for DNA damage and cell toxicity
When I started researching the mRNA jabs I actually started with the LNPs since that was easier to understand based on my training and knowledge base. Here is a brief summary of LNP characteristics that are often not understood.
They are big, relatively speaking. Using the term nanoparticle makes it appear they are very small but they are in fact, about the size of a virus. It appears to the body similar to a cholymicron (a lipoprotein molecule) due to the synthetic fats. This is much larger than any drug or peptide or antibody or even vaccine currently in use. The only exception are pegylated nanoparticle medicines like pegylate liposomal doxorubicin (chemo).
They are spontaneously made using electrostatic forces. They are not chemically made in a step wise fashion.
As a result, they are fundamentally unstable and over time (months or longer, maybe less depending), they grow bigger and fuse together or agglomerate. This is known as the Oswald ripening Oswald ripening. Likely one reason they need to be frozen. If they get too big ( like >500nm) then you can get embolisms and clots. (sound familiar?).
They are an amorphous mass. Those cartoon pictures you see of LNPs with a bilayer and water and mRNA inside? Totally wrong. They are more solid with little water.
They have a slight negative charge. This, of course can change if they agglomerate, or because of buffers or other reasons.
Process 1 LNPs were about 60-80nm in size. Process 2 LNPs ranged in size from 60-180nm. Nanoparticles larger than 200nm (just because they are nanoparticles, NOT because of the lipids specifically) can activate the complement system which results in CARPA (complement activated-related pseudo allergy). This requires a substack entry by itself. Here is a great review article. CARPA and the ABC phenomenon
Loss of mRNA activity between the bulk drug substance and the final mRNA-LNP product
In order to determine biological characterization (what the EMA calls it), cells are transfected and the desired protein is expressed which is further characterized via Western blot for size. (BTW no amino acid sequence has been performed for the protein expressed by the mRNA jabs)
This analysis, called in vitro expression, or IVE, was done on the bulk drug substance after the mRNA is produced and purified.
However, when the same analysis was performed using the actual drug product a drop in the IVE was seen. On average it was about 5% but could be much higher than that. THIS DROP WAS NOT PRESENT IN PROCESS 1 LOTS.
It was determined that this was due to lipid impurities. This is from the published European Public Assessment report by the EMA. EPAR
Lipid-related impurities have been identified in the finished product and have been characterized. An investigation has been initiated and is ongoing to assess and review potential root causes. The outcome of the investigation shall be provided (SO2).
These lipid related impurities were considered important enough, that a Specific Obligation was laid down to determine the cause and to fix it.
Late Migrating Peaks (LMPs)
The lipid impurities were first identified in the determination of RNA integrity assay using capillary gel electrophoresis (CGE). Here is an example of one of the earliest Pfizer lots EJ1685.
You can see the RNA fragments (truncated and fragmented mRNA) which are smaller than the intact mRNA strand. Then you have a bump AFTER the main mRNA peak which represents these LMP or as the FDA called them, Late Migrating Species (LMS).
FDA Report on Pfizer’s LMPs
In its review of the Pfizer vaccine, the FDA did ask the following question in late November 2020.
QUERY 6 (FDA)
Please state the percentage of DP lots that have the LMS peak and provide a list of all impacted DP lots, including information on the DP manufacturing site as well as the associated DS lots and lipid lots/sources used for DP manufacture.
Here they are trying to find out, what is causing these LMPs and exactly what are they? The prevailing theory is that perhaps some lots of lipids or even of the drug substance was causing these issues.
Here is how Pfizer answered the question (see previous image).
The RNA integrity assay reports the % time corrected area of the main peak, with all other peaks (RNA fragments preceding main peak and LMS RNA species trailing main peak) influencing the reported % RNA integrity value. Most lots (14 out of 20) have some level of late migrating species reported.
The release specification for RNA integrity controls both RNA fragments and LMS since both of these species lead to lower RNA integrity. The release specification limit has been tightened to ≥55% to ensure the integrity of RNA is maintained through the point of use. For lots that meet release specification acceptance criteria, LMS ranged up to 16%.
Holy Toledo! Upto 16%!! So notice that the bulk drug substance must show >55% full intact mRNA integrity so that at the end of the manufacturing process there is at LEAST 50% intact mRNA in the LNPs. This is how they solved the issue for EUA authorization. Just change the acceptance criteria.
Changes in LNP Manufacturing Between Process 1 and Process 2
As per the rolling review
Changes to accommodate scale-up and facility fitting have been made; for example, the aqueous and organic phase concentrations and flow rates for LNP fabrication were changed from a “classical” process to an “upscale” process to enable higher process throughput, combined with “scale out” flexibility of parallel processing using multiple T-mixers. The scale of the tangential flow filtration (TFF) equipment was similarly adapted for larger batch volumes and higher process throughput.
Here are the basic changes, most have to do with new manufacturing sites, and new manufacturers. Now that wouldn’t cause a problem, would it?
So the FDA asked Pfizer to look and see if there were differences between LNP manufacturers or the lipids themselves. Remember that the ionizable lipid ALC-0315 and the pegylated lipid ALC-0159 were NOT PHARMACEUTICAL GRADE. Could this be the problem??
My brief assessment says there may be lots of the ALC-0315 which are more prone to forming the LMS than other lots but it is not consistent. Maybe it was manufacturing at Croda which was brought on when they upscaled the lipid manufacturing??
Moderna Finds the Cause
I have to hand it to Moderna scientists. They found the cause of the loss of mRNA activity from the bulk drug substance to the final product. This was submitted in June 2021 and published in Nov 2021. So they were working on this from late 2020.
Novel mechanism for mRNA loss in LNPs
I just want to highlight the major takeaways that I found important.
it is a NOVEL mechanisms, which means this was a surprise to Pharma
it was not picked up with normal analytical techniques. So important. Only when they used more precise analysis of the lipids using IR-RP-HPLC instead of regular HPLC did these lipid aggregates show up. And we were told these LNPs were studied for decades. Pffft.
It made the mRNA untranslatable. You can’t have that or you don’t get enough antibodies schmantibodies.
It was due to the ionizable lipid, i.e. ALC-0315 or in Moderna’s case SM-102. So broadly applicable to all tertiary amine ionizable lipids.
It was due to the formation of reactive species including oxidation and subsequent hydrolysis of the tertiary amine of the ionizable lipid. A chemical reaction.
This chemical reaction form aldehydes which then form covalent adducts of the reactive lipid species to the nucleobase of the mRNA. This is a permanent bond which destroys the mRNA backbone.
Moderna scientists did not speculate on WHAT THESE ADDUCTS COULD DO INSIDE THE CELL. It is a well known fact that aldehydes can be mutagenic.
If adducts are formed with the mRNA, can they also be formed with the DNA found in these shots? Is it specific to the mRNA nucleobase? Can anyone comment?
Here is a recent review of aldehyde associated mutagenesis.
Aldehyde associated mutagenesis
At the molecular level, aldehydes damage DNA, cross-link DNA and proteins, lead to lipid peroxidation, and are associated with increased disease risk including cancer. People genetically predisposed to aldehyde sensitivity exhibit severe health outcomes.
Not good.
Hmmm aldehydes do cross link with DNA; one mechanism of genotoxins is linking of a protein and a DNA strand giving a DNA-protein adduct. Just like with mRNA adducts. Imagine that.
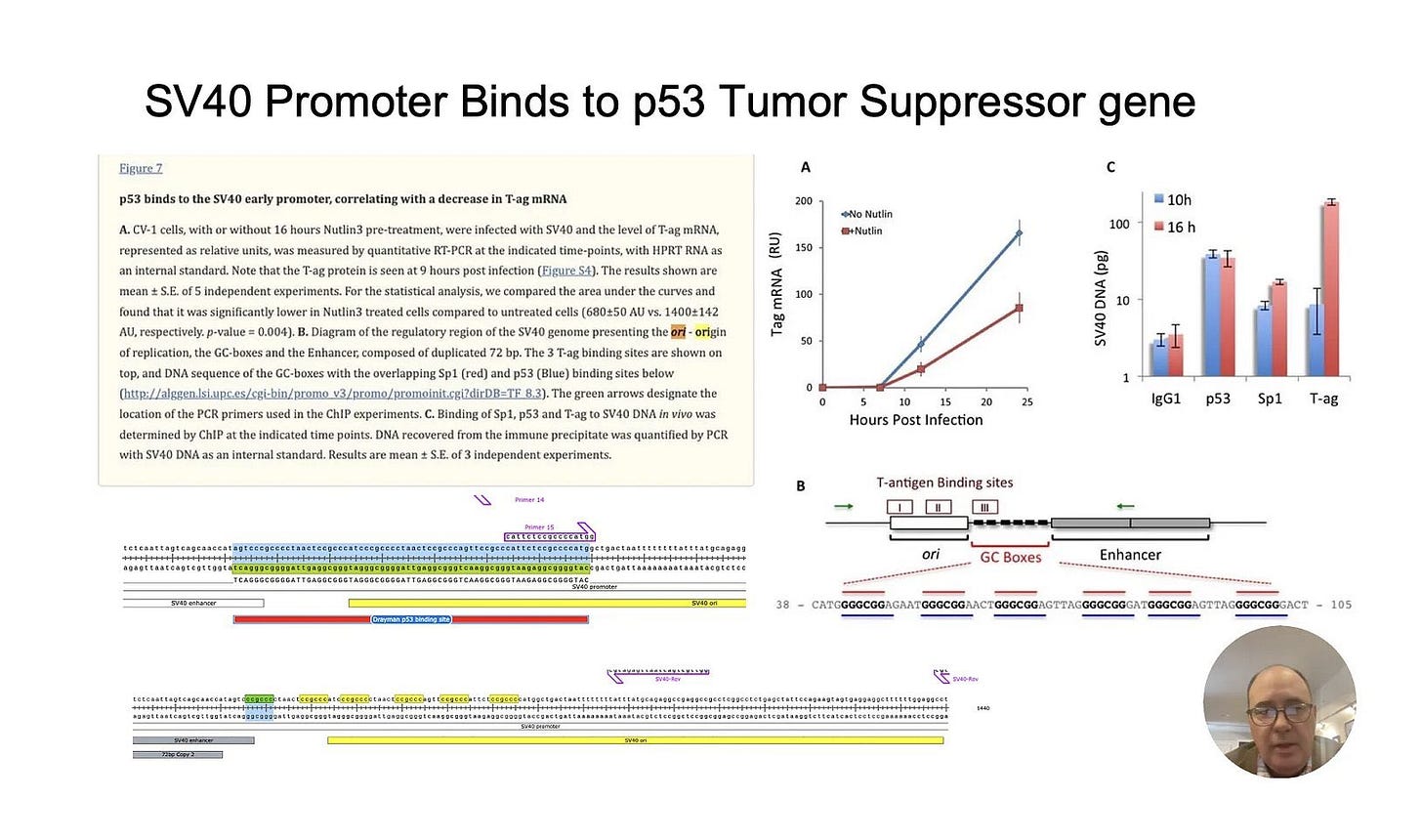
Then we have p53 which regulates the cellular response to DNA damage from aldehyde stress for example. And what does the the SV40 promoter in the vials do?
More: endogenous aldehydes and alcohol
Aldehyde Formation and mRNA Strand Breaks
It is always instructive to read what Pharma says about this stuff. Moderna presented its finding on LNP adducts in its Moderna Science Day in May 2022. Moderna ALWAYS had Tris as its buffer.
They also found this Late Migrating Peak or Species INCREASED OVER TIME AND WITH INCREASED TEMPERATURE. These adducts are formed because of acid-based hydrolysis and acidic buffers used in the formation of the LNPs.
So why are adducts a problem? Because the mRNA is now nonfunctional and there are mRNA strand breaks. What about the DNA in there?
Well look at this. The TRIS BUFFER ACTS AS AN ALDEHYDE SINK. I think this is why Moderna’s product did not need to be stored at -70C compared to the Pfizer product. Pfizer used the PBS buffer. Within 10 days in the fridge the % adducts almost doubles for both manufacturers though that stabilizes with Moderna.
What Caused These Aldehydes to Form and How to Fix It?
Moderna proposed that the control of oxidation of the lipids and increased purity of the lipids would decrease adduct formation. Impurities such as?
When the mRNA drug substance or drug product is stored at neutral pH and frozen, degradation caused by depurination and deamination has been found to be of low risk with degradation rates being extremely low; therefore, no control strategy is required. In contrast, oxidation of the bases in the mRNA, as caused by oxidation reactions often catalyzed by redox-active metal residues (iron, copper, etc.) or promoted by light exposure, are more likely to occur. These impurities, often associated with the lipids in the LNPs, are a potential risk for degradation of the mRNA and require analytical control strategies
Storage and stability of mRNA vaccines
Well we now know about aldehydes. But METALS??? Are they in the lipids?Here is what the EMA found with regards to Pfizer but I am fairly certain it is the same with Moderna.
Ohhh I see copper, cadmium, arsenic, cobalt, vanadium and lead for example. All under the “acceptance criteria” of course. Plus people have copper and iron in their blood. Metals act as a catalyst and can accelerate base degradation. So the risk of aldehyde formation remains, imho.
Pfizer tried to justify that the impurities in the lipids were acceptable.
Just let this go EMA, OK? It’s a PANDEMIC.
Pfizer Changes to Tris Buffer
Well Pfizer DID have to improve re LMPs. We are lead to believe that the change to a Tris buffer in October 2021 from Phosphate Buffered Saline (PBS)/sucrose for increased stability, simpler storage requirements and to provide a ready-to-use formulation that did not require dilution at the time of administration. There was NO COMPARABILITY TESTING ON HUMANS OR ANIMALS OR PHARMACOKINETICS, PHARMACOLOGY OR TOXICITY ASSESSMENT. But the major reason were these adducts IMHO. If there were less adducts, less mRNA strand breaks, more available mRNA would that not change dose? Or the amount of intact mRNA available for translation? Decreased toxicity? I call this the Process 3 change because it eliminated those difficult to prepare purple top vials and I suspect these early PBS buffered jabs were more toxic than the Tris containing ones. And it is not just about aldehydes. Guess that is another substack.
Eliminated the LMPs Was More Important Than Purifying the mRNA
Both Moderna and Pfizer worked hard at purifying their lipids in order to get rid of the LMPs. How do I know? Because the lipid manufacturer themselves told us. MilliporeSigma
The fact that mRNA is prone to degradation means that any impurities within the lipid excipients used to encapsulate it should be considered for their impact on the performance of the mRNA overall. Therefore, impurities in lipid excipients must be controlled very tightly and regulatory parameters are very stringent.
BUT Pfizer did not improve the levels of residual DNA and Moderna still has issues with dsRNA which they are trying to fix…..maybe.
WHY? WHY? WHY?
Why did Pfizer and Moderna clean up the lipids but did almost nothing for mRNA purity?














Holy Toledo! You are brilliant. I'm going to have to go thru this post with a fine -tooth comb!
I am aware genetically that I am sensitive to aldehydes. Fortunately, I'm really well protected in oxidative stress vs aggression!
Thank you this this article! My office manager wants me to decrease my number of paid Substack. I told him simply Not Geoff Pain!! 😁
Very clear explanation. Better than from some so-called LNP "experts."
Thank you!