I have been digging into Process 3 for about 2 years now. Process 3 is what we are calling the change from the PBS buffer to the Tris buffer by Pfizer. The change occurred in October 2021.
Here is the background
To that end I filed an ATIP on Jan 30, 2024 regarding HC issuance of an NOL (No Objection Letter) regarding the change to drug manufacturing of Comirnaty specifically. No residual DNA levels since that is measured upstream when the mRNA is made. Here I am looking at the final drug product.
This is what I asked for
Please provide the following data regarding the issuance of an NOL (NoObjection Letter) on 2021-10-16 NC# 257449 regarding changes to the drug manufacturing process of Comirnaty. Data regarding bench top comparability between the previous phosphate buffer formulation and the newer formulation containing the Tris or tromethamine buffer of Comirnaty with respect to
LNP size; zeta potential; RNA concentration; lipid aggregates and lipid
impurities; capped intact RNA; in vitro expression; and endotoxin
The results and review of the comparability assessment of the original formulation in purple top vials versus grey topped vials with respect to actual or potential toxicity, and change in available mRNA content.
Yesterday I received my data.
All 46 pages are like this. This is the reason why in both of Canada’s official languages.
20(1)(b)
Subject to this section, the head of a government institution shall refuse to disclose any record requested under this Act that contains (b) financial, commercial, scientific or technical information that is confidential information supplied to a government institution by a third party and is treated consistently in a confidential manner by the third party
Le responsable d’une institution fédérale est tenu, sous réserve des autres dispositions du présent article, de refuser la communication de documents contenant : b) des renseignements financiers, commerciaux, scientifiques ou techniques fournis à une institution fédérale par un tiers, qui sont de nature confidentielle et qui sont traités comme tels de façon constante par ce tiers
So there you have it.
This data is becoming even more important because of the results of this recent study available here
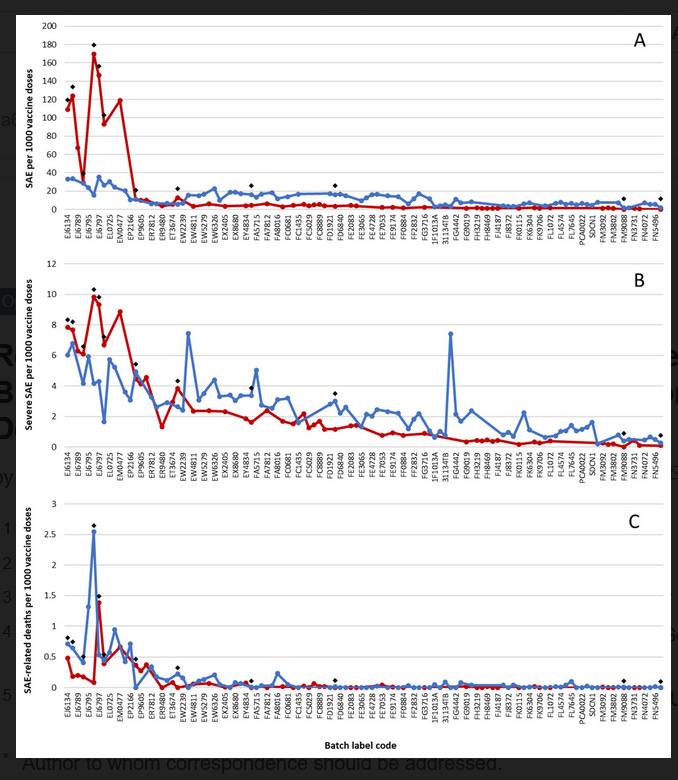
Here is what they showed by Lot Number. The earlier batches had more AEs than the later batches. At what Lot Number they changed to Tris and whether they are the same in Europe or elsewhere is unknown. There are a ton more variables of course, and the manufacturing plants may produce different products between the EU and NA or other sites. Remember, the process is the product.
Also, the observation that there are MORE overall adverse events with the early lots but not necessarily severe adverse events is in keeping with acute systemic or anaphylactoid (CARPA) reactions which are not anaphylaxis (no lip edema and no respiratory distress) but you can have a red rash, tachycardia, and hypotension etc most are not long lasting but occasional deaths have been reported with CARPA. You can read more about CARPA from the excellent work by the world expert Szebeni and his group here. BTW they used lot ET7205, an early lot for Pfizer in these experiments.
This is my current working hypothesis, subject to change with more data.
Also there could be issues from the particle size and the lipid adducts.
Oh and this also is in keeping with the authors previous work (Schmeling et al) in Denmark here
The truth will come out.
Pray the rosary









The more they redact, the more citizens smell rats.
You might like
https://geoffpain.substack.com/p/tromethamine-is-a-hazardous-substance
and
https://geoffpain.substack.com/p/carpa-jab-anaphylaxis-caused-by-endotoxin
Tromethamine was used by Moderna from the outset in its Covid19 Jabs to scavenge Aldehydes that form adducts with DNA and mRNA
See also
https://geoffpain.substack.com/p/acetaldehyde-from-ethanol-in-pfizer