The Biodistribution/Metabolism/Excretion of the Lipids, Part 3
Continuation after biodistribution including transfection, translation and excretion of the LNP components; and just a little ranting. OK a lot of ranting.
Well this substack ended up being much longer than I expected, but I wanted to show what I found when you ask the questions one would normally ask regarding the basic kinetics and pharmacology of a new drug or therapy. The focus on antibodies, schmantibodies has obscured the big black box on data regarding the mechanisms from administration to spike protein formation. I think it is because many of us do not realize these jabs are PRO-DRUGS and as such should the assessed with this in mind.
Recap
What we learned in Part 1
traditional pharmacokinetics not appropriate for LNP/RNA products
I propose instead of Abosorption, Distribution, Metabolism and Excretion (ADME), we should use Absorption, Biodistribution, Translation/Transfection and Excretion (ABTE)
Moderna’s draining lymph nodes was likely the source for “it stays in the arm” since it was the only IM biodistribution study done preauthorization but is deceptive and incomplete.
The biocorona on the LNPs defines its biophysical identity and interactions with cells
What we learned in Part 2
what is required for an appropriate biodistribution assessment of LNP/mRNA products
thus the Pfizer biodistribution study doesn’t tell us much
what current and future regulations will require which was not performed for these products
an analysis of a mostly complete biodistribution study of Lipid 5 LNPs by Moderna, but given IV
LNP distribution
broken up LNPs and mRNA
mRNA in cytosol
protein expression
These elements must all be measured and the relationship between LNP/mRNA distribution and mRNA in the cytosol or protein production is not always correlated.
Di et al mRNA LNP uptake does not necessarily correlate with mRNA protein expression This is a must read article because it clearly shows the effect of size, route of administration and levels of expressed protein. They conclude:
The LNP exposure and the transgenic protein level were not linearly correlated. This non-linear relationship between the LNP exposure and the protein expression level varies in different tissues and organs. In addition, as shown in Fig. 6, even when more small particles could accumulate in the liver than the medium particles, the luciferase activities in the liver did not become stronger. Such difference could result from the size preference of endocytosis by cells. (or by saturation, Michaelis Menton kinetics?/ed)
WHEREIN I RANT: the biodistribution is not a general overall exposure of the LNP/RNA particles in the body, like we see with drugs and resultant immunologic responses, for example. The exposure to LNPs is tissue/organ specific (due to size and fenestrated epithelium, see below) and sometimes even focal within an organ or tissue. There does not appear to be any known traditional factors to date that affect biodistribution such as age, obesity or dose. Anyone have data on this? In addition, exposure to mRNA and protein expression has been found in tissues and organs not usually exposed or transfected by LNPs as shown in the studies reviewed. WHAT GIVES? HOLD THAT THOUGHT. IT’S IMPORTANT.
Some Additional Notes on Biodistribution
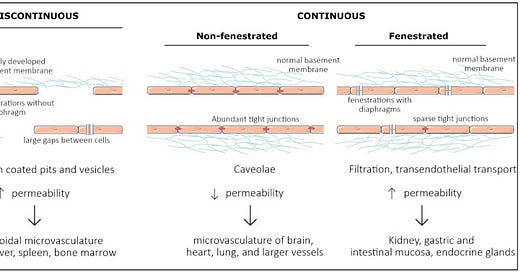
FENESTRATED EPITHELIUM
Vascular endothelial cells line the entire circulatory system and show remarkable heterogeneity. There are 3 types of endothelial cells: discontinuous, fenestrated, and non-fenestrated endothelium (see below). These morphological differences correlate with vascular permeability and contribute to organ-specific functions. Non-fenestrated endothelium has a low permeability, which is found in brain, heart, and lung microvessels, as well as within all larger vessels (i.e., arteries and veins).
A fenestrated endothelium has transcellular pores of about 70 nm in diameter, which are covered by a thin, non-membranous diaphragm. As fenestrae are associated with increased filtration and transport functions, they are found in kidney, endocrine glands, and gastric and intestinal mucosa.
Discontinuous endothelium is found exclusively in sinusoidal endothelium, such as the bone marrow, the spleen, and the liver endothelium. The latter has larger fenestrations, up to 100 or 200 nm in diameter, that are devoid of a diaphragm and have large pores within individual cells resulting in a high permeability.
Each organ is made up of different types of endothelia. In the kidney, fenestrated endothelium in the peritubular capillaries and glomeruli ensures proper filtration, while continuous endothelium elsewhere provides the kidney itself with nutrients and oxygen. Similarly, circumventricular organs of the brain are lined with fenestrated endothelium, while elsewhere, the tight blood–brain barrier (BBB) is found.
Representation of the three main structural phenotypes in organ-specific microvasculature. Discontinuous endothelium is mainly found in the sinusoidal microvasculature of the liver, spleen and in bone marrow, and is characterized by large fenestrations and pores within and in between endothelial cells, respectively. It has a poorly developed basement membrane and contains clathrin-coated pits and vesicles that dramatically increase permeability. Non-fenestrated endothelium is characterized by low permeability and a high abundance of tight junctions and caveolae. It is mostly found in the microvasculature of the brain, heart, and lung and in larger vessels. Fenestrated endothelium has an intermediate permeability and is characterized by fenestrations covered with a diaphragm. These fenestrations and sparse tight junctions ensure proper filtration and transendothelial transport, as found in the microvasculature of kidney, gastric and intestinal mucosa, and endocrine glands.
So how big are the LNPs? Well for Process 1 jabs, they were mostly 60-80nm so could easily penetrate the kidney, gastic and intestinal mucosa and endocrine glands like the thyroid and adrenals. For Process 2 jabs, they ranged in size from 60-200nm so can still slip in easily to the liver and spleen, and in the bone marrow. And the smaller ones squeeze through the the endocrine glands, kidney etc.
In organs with a continuous endothelium, LNPs must pass through the endothelial cells to reach other types of cells. This transendothelial delivery of LNPs occurs mainly through transcytosis (ie the mechanism for transfection) and is usually inefficient. So the administration of the LNPs in the blood vessels and endothelium does NOT mean rapid uptake by vascular endothelium via transfection, based on what data is available.
For example, after intramuscular administration of an mRNA LNP influenza vaccine to mice (Moderna, natch), branched DNA analysis of tissue mRNA identified muscle > lymph node > liver > spleen > ileum > bone marrow > testes as the primary organs of LNP accumulation in descending order, in keeping with fenestrated epithelium, imho.
So how does the knowledge of fenestrated epithelium inform the biodistribution of the lipid nanoparticles in the Pfizer study and in the recent Moderna study of Lipid 5 in Part 2?
I will let the reader decide.
PARTICLE SIZE/CHARGE AND BIODISTRIBUTION
Other commentators have expanded on the particle size and charge and how that relates to biodistribution so I won’t expand upon this. However, these factors must take into account the influence of the bio corona and distribution through fenestrated epithelium.
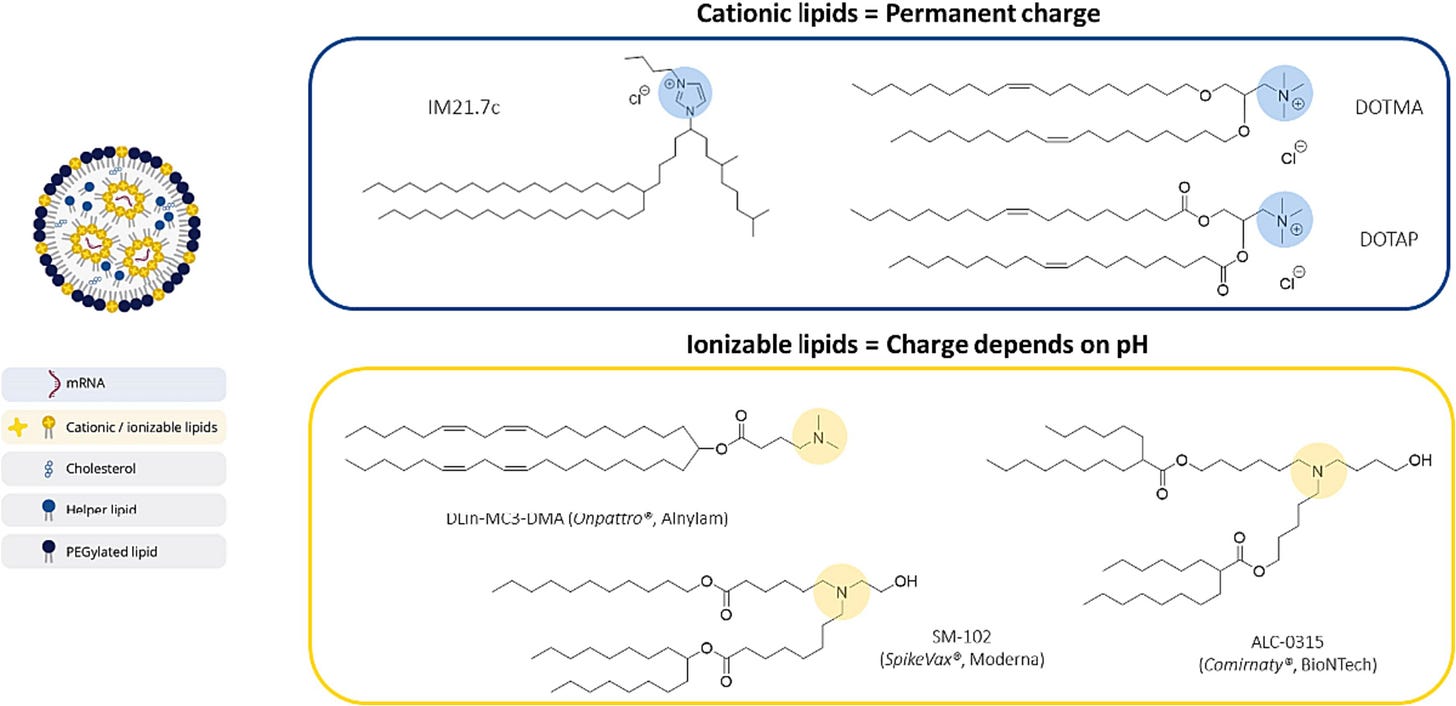
LNPs comprise four key elements:
an ionizable lipid Pfizer=ALC-0315 and Moderna SM-102)
helper lipid (DSPC),
cholesterol (ordinary regular cholesterol)
and poly(ethylene glycol) (PEG)-functionalized lipids.
Ionizable lipids typically exhibit a pKa < 7 and are therefore deprotonated (neutral) during circulation, which enhances the safety profile of lipids compared to permanently cationic lipids.
WHEREIN I RANT: There is a difference between permanently charged cationic lipids and the ionized lipids used in the jabs and other gene therapy products. NO ONE is actually using the permanently charged cationic lipids in humans because they are TOO TOXIC and almost non biodegradable (complement activation and cytokines galore). So all the size, charge and distribution studies using these cationic lipids do not inform us on the safety, efficacy or biodistribution of the currently used ionizable lipids. So I usually ignore any of these papers, unless they are comparing them to ionizable lipids. Here is what they look like and where the permanent charge resides.
The protein expression difference between a cationic lipid IM21.7c (blue) and an ionizable lipid DLin-MC3-DMA (yellow). Enough said.
Here is why ionized lipids are safer, since 2013.
The Case of Onpattro
In my many years of analyzing new drugs and vaccines for pricing review, I would always look to see if there were any similar or comparable drugs or compounds, or anything that was used for the same indication as the drug under review. Well in the original European Public Assessment Report (EPAR) for the Pfizer vaccine, they mentioned this drug Onpattro which was approved in the EU in 2018.
Onpattro (patisaran) is a product to treat hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis), a rare life-threatening disease. This is caused by mutations in the TTR gene causing polyneuropathy and cardiomyopathy usually diagnosed in males in the 7th decade.
Patisaran (Onpattro) is an LNP product using DLin-MC3-DMA as the ionizable lipid (see above), PEG-2000-DMG and the same cholesterol and helper lipid DSPC as found in the mRNA jabs. The “active ingredient” is small interfering RNA (siRNA) which is a 19nt dsRNA that reduces the the expression of mutant and wild type transthyretin (TTR) mRNA and its corresponding protein. Onpattro is targeted to delivery to hepatocytes in the liver (the PEG sloughs off and the LNP gets covered with ApoE, haha), the primary source of TTR protein in the circulation. The siRNA is chemically-synthesized (so NO plasmids!!!) double-stranded oligonucleotide.
A full pharmacokinetic and biodistribution assessment was done as was safety pharmacology, toxicology, carcinotoxicity, genetotoxicity, and reproductive toxicity assessments were done. Most importantly an ENVIRONMENTAL RISK ASSESSMENT (ERA) was done, in keeping with the EU Guidelines for all drugs used for human use. (So how come this was not done for the mRNA jabs?????) This includes screening for persistence, bioaccumulation and toxicity and “shedding” into the environment, because this is a genetic therapy product. Because this disease is so rare and the dosage is relatively low, the overall risk to contamination to water and the environment was considered low. Now think about what an ERA for the mRNA jabs would look like.
So one can see how the regulatory assessment of Onpattro helps us with
biodistribution/kinetics
mechanism of action in the cell
elimination of the individual components of the LNP/siRNA product
toxicities of the LNP and ionizable lipids, potential adverse events, etc
“shedding”
Transfection of nucleic acids or how the LNPs cross the cell membrane and what that does
What we know about how the LNPs of the mRNA jabs cross the cell membrane, how the mRNA is released and how the mRNA is translated to the spike protein comes from the studies of Onpattro. You probably have seen a version of this as an explanation of the metabolism and pharmacology of the LNPmRNA particles intracellularly. The Onpattro story gives an easy to read history of how this product was developed, particularly the LNPs and is worth the time reading this article.
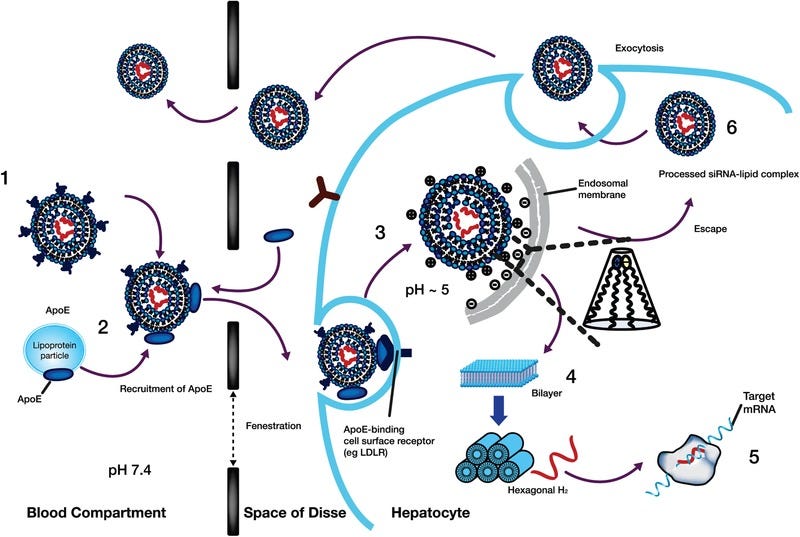
OK here you can see for Onpattro (patisaran)
the PEG on the LNPs slough off and are replaced with ApoE
the ApoE covered LNPs go through the fenestrated epithelium of the liver
the bio nature of the LNPs facilitate its using endocytosis into an endosome
the pH of the endoscope is about 5, allowing protonation of the LNPs, destabilization of the LNPs and the endosomal membrane releasing the siRNA
the siRNA goes to the RNA-induced silencing complex and does its thing in silencing the gene making the transthesic protein.
So is this happening with the mRNA products? How many cells are transfected with the LNPs? Which tissues? Where is the data?
HOW EFFICIENT ARE LNPS AT TRANSFECTION ORGANS AND TISSUES? As it turns out, not that efficient at all.
Perturbations of the cell membrane (or transfection is EVIL)
Something that is absolutely critical to understanding these products is to understand that transfection of nucleic acids is a totally new paradigm of delivery a biologic active substance to a cell. TRANSFECTION IS LIKE A KNIFE OR A STEALTH BOMB. Because the LNPs have a biological identity, they appear like a biological particle but once endocytosed into the cell, then the synthetic biology of the lipids and mRNA is recognized by the cell. This is much different than small molecular drugs or even therapeutic proteins since these molecules exert their effect through ligands, receptors or ion channels imbedded in the lipid bilayer of the cell. The cell knows what it is dealing with and sometimes regulates whether drugs or chemicals are allowed in (like tumors becoming resistant to chemo etc). Information and signalling starts from the outside in (usually, as always there are exceptions like maybe general anaesthetics). However, these LNP/mRNA particles START from the inside; in essence bypassing all protective and sensing ability of the cell membrane.
Moreover, these ionizable lipids have an accentuated cone-shape molecular structure which have shown to destabilize the lipid bilayer of the cell membrane. Just the fact that ApoE is adsorbed to the LNP causes rearrangement of the LNP component INSIDE THE PARTICLE which affects its nanostructure and RNA delivery to the cells. How complicated is that? And are these ionized lipids incorporated into our lipid bilayer? These lipids are like trans fatty acids on steroids. Is cell membrane fluidity lost?
Honestly this stuff is so complex AND THERE IS STILL A LOT OF UNKNOWNS. If you really want to know how delicate and complex the lipid bilayer of our cells really are, and what transfection with these synthetic lipids do, then I highly recommend reading Genervter’s latest substack to get a general idea (even if a lot is in German, there are enough articles and studies in English). I wonder if a transfected cell will ever be the same again.
Release of the mRNA from lysosomes
It is well known, as shown with Onpattro, that the release of the mRNA from the lysosomes in the cell cytosol is the RATE LIMITING STEP of the mRNA jabs.
The precise sites and mechanisms whereby LNPs help mRNA to escape from the endosomal lumen are, to date, mysterious
You don’t say? IT IS A BIG BLACK BOX. No one knows how it happens, why it happens, how often it happens. This mechanism is still being worked out. Here is a schematic of a recent analysis of LNP-mRNA delivery
That mRNA and lipid particles do not readily release from the endoscope, despite the design of LNP/mRNA particles such as the type of synthetic lipid, the molar ratios, the pKa and other factors. So what’s happening here? Much of the LNP-mRNA accumulates in large endoscopes which do not acidify (well not enough), do not release the mRNA and are not available to make spike protein. THIS LEADS TO LNP TOXICITY.
These researchers suggest that if the endosomal maturation arrests and there is accumulation of undegraded cargo (ie the mRNA and DNA as well as the broken down LNPs), then this is reminiscent of lysosomal storage disorders. Lysosomal storage disorders are rare inborn errors of metabolism genetic diseases. There are over 50 of them but the more well known are Gauchers disease , Fabray’s disease and Newman-Pick’s disease. The clinical consequence of substrate storage shows up in multiple organs and systems and is associated with visceral, ocular, hematological, skeletal, and neurological manifestations. I wonder is this is a possible explanation for some of the more puzzling neurological manifestations in particular, of vaccine injury? This theory needs more eyes.
Translation to Spike Protein
OK, let’s assume the mRNA is released and makes it to the ribosomes. How much and how long does translation last?
This interesting article looked at this question in HuH7 cell line and by single cells. They state that expression rate of protein DEPENDS ON THE CELL LINE and might depend on the STATE OF THE CELL, such as where the cell is in the cell cycle or on cell culture history. mRNA transfection was measured at the single-cell level. The distributions reveal that the delivery process occurs over a time period of only a few hours (see Figs 2C and4A) and is dependent on fetal bovine concentration which of course has proteins etc that form the bio corona. And that NOT ALL CELLS are transfected even if they are beside each other. They also find, in agreement with the literature, that there is only a small window of opportunity for successful carrier release before the nucleic acid nanocarriers are trapped in late endosomes or lysosomes. Is it possible that this finding that translation of the protein may depend on the cell cycle may explain some of the variability we see?
Of note, in the recent Bitounis article in Nature regarding reducing LNP toxicity he states that ONLY 1% of the total LNPs eventually getting translated, what happens to the rest of the LNPs?
I don’t know how Bitounis came to that number but there are a LOT of LNPs in each dose, estimated to be 10-40 billion, so 1% is still at lot, but this highlights the issue of the release of the mRNA (oh and DNA) from the endosomes. I believe that a substantial proportion of the entire LNPs are excreted through the kidneys and via enterohepatic circulation in the liver via the faces. This is shown to a certain extent in the Lipid 5 trial shown in Part 2. I also believe that because of the electrostatic interactions that form the LNPs this results in instability in the blood stream. Shearing stress, pH changes and increased temperature may indicate that some of the LNPs are broken up within the blood stream or in organs. Of course this may result in inflammatory or other reactions that needs further study.
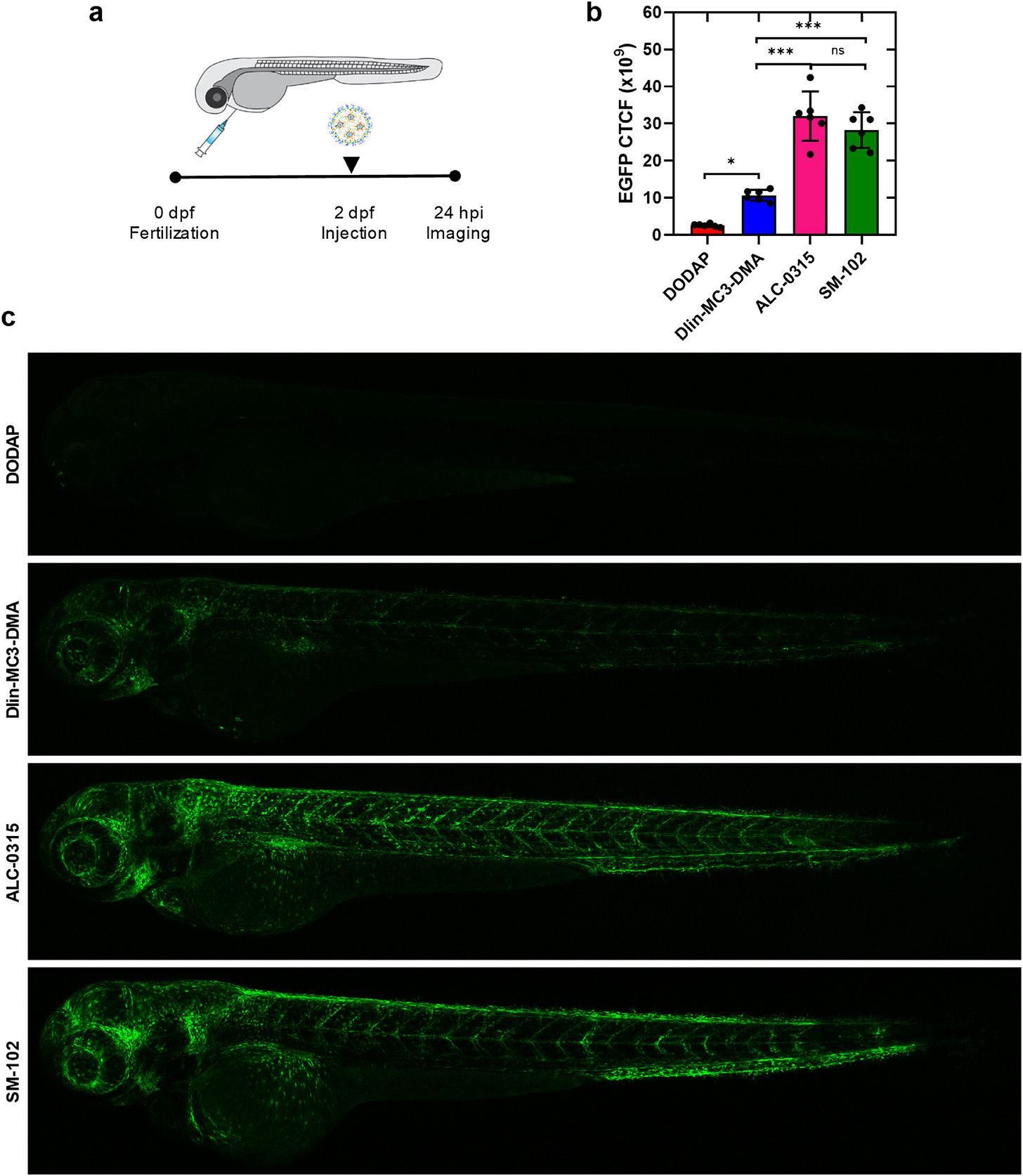
This study of in vivo vs in vitro evaluation between transfection and vaccine efficacy is important because it answers the question whether the dose of Moderna at 100ug vs Pfizer at 30ug results in more protein expression or increased antibody levels.
Fig. 5. In vivo mRNA translation of EGFP-mRNA-LNPs in zebrafish embryos. (a) Schematic illustrating the intravenous injection of the EGFP-mRNA-LNPs in wild-type ABTL zebrafish embryos (2dpf). (b) CTCF fluorescence intensities obtained after injecting the EGFP-mRNA-LNP formulations to zebrafish embryos (EGFP-mRNA dose: 0.3 mg kg-1; injection volume: 1 nL), as determined by confocal microscopy and image analysis. c) Confocal microscopy images of the zebrafish embryos treated with DODAP, Dlin-MC3-DMA, ALC-0315 or SM-102. Data are shown as mean ± SD, n = 6; *p < 0.05, **p < 0.01 and ***p < 0.001.
Here you can see in zebrafish that both the Pfizer and Moderna vaccines results in similar expression of EGFP protein expression. So this likely demonstrates that the amount of mRNA in each dose is less important for protein expression than the factors influencing transfection, release from the endosomes and then translation into protein.
We still do not know what human tissues are more likely to be transfected than others (though I think muscle doesn’t get transfected that easily) and for how long the mRNA lasts (60 days longer) or how long the spike protein is present before breaking down (a year or longer?)
Exosomes, Schmectasomes
This is where the data on studies in Onpattro informs us regarding the ELIMINATION of the LNP/mRNA particles.
Because the regulatory review of Onpattro was well studied, this review article of Onpattro pharmacokinetics tells us what is likely happening with the mRNA jabs. Remember the ionizable lipids, PEG etc and molar ratios are similar.
The Pharmacokinetics of Onpattro (patisaran)
In the first 4 hours after dosing, the plasma concentration‐time profiles of the siRNA (ALN‐18328) and DLin‐MC3‐DMA (the ionizable lipid) showed a similar PK distribution phase, in which decline was more rapid than that of PEG2000‐C‐DMG (data on file). As the siRNA (ALN‐18328) and DLin‐MC3‐DMA ionizable lipid) are associated with LNPs, the rate of decline in their concentrations can be explained by the rapid uptake of LNPs by the liver (sound familiar?)
Imaging studies have shown that only a small fraction of the siRNA in the LNP is released into the cytosol and that about 70% of the siRNA that enters hepatocytes undergoes exocytosis through egress of LNPs from late endosomes/lysosomes back into the circulation. We propose that the second phase of the PK profiles for plasma siRNA and the ionized lipid corresponds to the exocytosis of the siRNA‐lipid complex from hepatocytes back into systemic circulation (Figure 5) but not as intact LNPs
Proposed mechanism of liver uptake of patisiran LNP and release from the liver following intravenous administration. (1) After intravenous administration of patisiran, PEG2000‐C‐DMG dissociates from the LNP. (2) Removal of the PEG coating allows endogenous ApoE to associate with the LNP, facilitating uptake into hepatocytes via an ApoE‐dependent process. (3) On internalization via endocytosis, the ionizable DLin‐MC3‐DMA lipid is protonated (positively charged), as the pH decreases in the endosome. (4) The positively charged DLin‐MC3‐DMA lipid interacts with the negatively charged endosomal lipid, resulting in disintegration of the LNP, destabilization of the endosome membrane, and release of ALN‐18328 into the cytoplasm. (5) ALN‐18328 binds to RISC, leading to the targeted degradation of TTR mRNA and subsequent reductions in the target protein levels. (6) A proportion of internalized LNPs undergo exocytosis egress from late endosomes/lysosomes back into the circulation.
IMHO, this explains much of what we are finding when we look at biodistribution studies and at mRNA and spike protein in the blood and tissues. Is it free mRNA and spike protein, or are they within exosomes?
Do we have any evidence that this is happening with the jabs. Well yes, we do. Bansal et al published in 2021 that the spike can be found in exosomes. And these exosomes were found at upto 4 months! It could have been longer but this was not tested.
(A) Representative NanoSight image for exosomes from vaccinated individuals with mean and median sizes (black thin line in the graph indicates the three measurements of the same sample, and red line is the average of all three lines). (B) Transmission electron microscopy images of SARS-CoV-2 spike Ag on exosomes from control exosomes from control and vaccinated individuals. Arrows indicate SARS-CoV-2 spike-positive exosomes. Right side, third image is the zoomed image of positive exosome from vaccinated sample (original magnification x 50,000). We have used anti-coronavirus FIPV3-70 Ab as negative control for both the samples.
Importantly, the data from Onpattro showed that exosomes from Onpattro had a half-life of 60-80 days! What does this mean? It means these exosomes could last upto a year if it takes 5 half lives to eliminate all exosomes. Exosomes are long lasting AND they could be the source of shedding.
Bansal also issued a commentary in 2022 to explain that they could not state that circulating exosomes generated after the first dose of vaccination persisted until 4 mo after the second dose of vaccine. This was after some of us noticed that this could explain the shedding phenomenon and that this was in keeping with gene therapy products like Onpattro. No one has followed up on these observations.
In fact, the extracellular vesicles as a form of vaccination of Covid with mRNA has been studied. Extracellular vesicles, which are surrounded by a membranous lipid bilayer, are released into the extracellular space by all types of cells for the transport of cellular cargo to regulate a variety of biological processes. So this is a way of not affecting the delicate lipid bilayer via transfection by using the bodies own lipid bilayer. Exosomes are characterized as the smallest EVs, ranging from 40 to 150 nm in diameter (oh the same size as the LNPS!!! how convenient) and originating from the endocytosis of the cellular membrane, resulting in the formation of multivesicular bodies (MVBs) by the inward invagination of the late endosomal membrane.
mRNAs encapsulated within EVs have been shown to be transferred to recipient cells and translated into proteins. So that means, all of these exosomes floating around for months CAN TRANSFECT OTHER TISSUES THAT WERE NOT TRANSFECTED BY THE LNPS THE FIRST TIME AROUND.
HOLY TOLEDO.
Plus, because these have a human lipid bilayer they do not cause the problems that transfection using LNPs cause.So the vaccinated don’t feel it as much. And it is likely the mRNA is released, or the spike protein and dissolved or broken up lipids. But would this would be sustained at a low level over time?
Finally, these exosomes are likely the cause of shedding AND THIS WAS KNOWN FROM THE BEGINNING BECAUSE THEY SHOWED THIS WITH ONPATTRO. Though the effect was not considered a risk because of the very low numbers of people receiving Onpattro.
WHEREIN I RANT. Why was the environmental risk assessment waived for the mRNA vaccines by the EU? IIRC, as special waiver was issues to avoid this requirement for the COVID-19 vaccines.
Please read my friend Helene Banoun’s review from 2022 (and she fought like crazy to get this published) and others who have written on this topic. To be clear, “shedding” in this context IS NOT SHEDDING OF THE VIRUS OR ANY COMPONENTS OF THE VIRUS, that can happen with other typical vaccinations (oh like live attenuate influenza vaccine which was the reason it was taken off the market). This is shedding of natural EXOSOMES with mRNA/lipids and in some cases the spike protein itself.
Putting it all together
This is what I believe happens (Maria’s hypothesis)
vaccine is administered IM (or IV) and the LNPs gets coated by proteins to make a biocorona
The LNPs get trafficked to the liver and other organs with fenestrated epithelium
Some of the LNPs are broken up in the blood stream and the mRNA/DNA/lipids are excreted through the kidneys and feces
The fenestrated organs (and the heart) are exposed to the LNPs and a substantial proportion of these are transfected
Many of the transfected cells do not release the mRNA but are stored in late lysosomes contributing to LNP toxicity
Some (or many) of the transfected cells eliminate the mRNA and maybe the spike protein via exosomes
Exosomes float throughout the extracellular fluid and transfects further tissues including eyes, brain, peripheral nerves? over several months. Hypothesis here
This is all happening at pretty much the same time; ie exosomes are forming as cells get transfected as the broken up LNPs get eliminated via kidney/feces.
At this point I haven’t considered what the DNA is doing and what the lipid adducts are doing, but we can think about this as well given this framework.
Variability between cells is HUGE so that some get almost no translation and others huge amounts; what governs this? Add the batch and vial issues which is easier to understand, but individual variation may be just as huge or greater. Possibility the manufacturing issues can be blamed for the wide variety in response but is only a partial explanation or a plausible deniability?
Lots to think about here when we ask questions.
Thank you for reading. Please share any comments or ideas regarding what I have found in this research and tell me how off base I am, LOL
Oh and pray the rosary.
a comprehensive review (neat article if you are interested)







https://genervter-substack-com.translate.goog/p/die-phospholipide-doppelschicht-und?_x_tr_sl=de&_x_tr_tl=en&_x_tr_hl=de&_x_tr_pto=wapp
Autotranslation should help a bit. :-)
Re: GP120 variable loop inserts. I thought I would test my hypothesis about cleaved S1 trafficking. This adds a few more concerns!
Extracellular Vesicles Carry HIV Env and Facilitate Hiv Infection of Human Lymphoid Tissue
...Here, we found that a significant number of EVs (exosomes) released by HIV-1 infected cells carry gp120 (Env), a viral protein that mediates virus attachment and fusion to target cells, and also facilitates HIV infection in various indirect ways. Depletion of viral preparations of EVs, in particular of those that carry gp120, decreases viral infection of human lymphoid tissue ex vivo. Thus, EVs that carry Env identified in our work seem to facilitate HIV infection and therefore may constitute a new therapeutic target for antiviral strategy.
...Biogenesis of these EVs inside the cells resembles that of retroviruses, in particular of HIV, and as a result, EVs share with these viruses many chemical and physical properties3. It is now understood that since EVs are released by HIV-infected cells, any HIV preparation is in fact a mixture of virions and EVs.
https://www.nature.com/articles/s41598-017-01739-8