Update
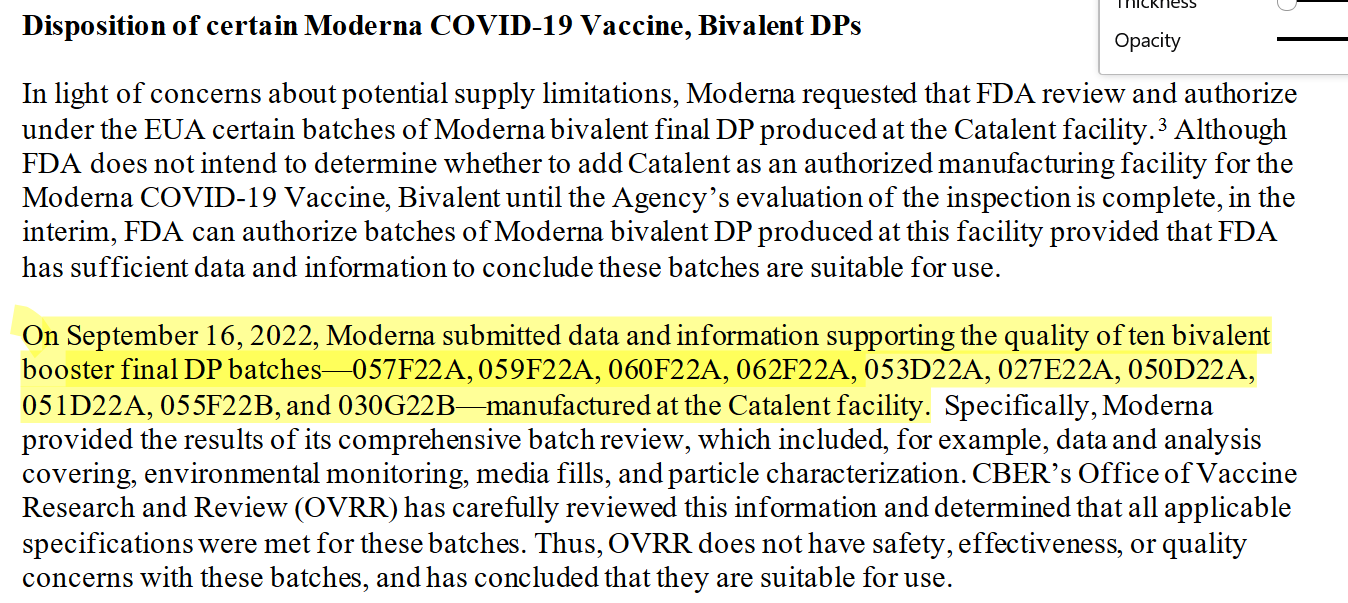
Eagle-eyed Jessica Rose noted that the Moderna batch 052D22A is NOT included in the batches identified by the FDA and released. However, batches 050D22A and 051D22A are mentioned so it is possible 052D22A wasn’t actually tested but I would expect to have the same issues as the other batches.
This also gives background as to these early booster bivalent doses Moderna's new booster launch tripped up by production issues at Catalent plant: reports
1. Evidence supporting early lots of the Pfizer vaccine were toxic than later lots.
Dr Peter McCullough just recently summarized the data supporting the increased toxicity of the early lots in the US.
I have noticed in my practice that nearly all of those with serious COVID-19 vaccine syndromes including myocarditis, blood clots, and other live-threatening problems received their first shots either in December 2020 or early 2021. Pfizer’s lots or batches have been evaluated and studied for variation in risk by Schmeling, Manniche, and Jablonowski. All three studies have concluded the earlier batches were more lethal and the variation in risk was considerable from lot to lot. Now Jablonowski and Hooker report:
Here is the paper. Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine in the United States. The conclusion states:
So batches allocated in the first 2 months, sent out to hospitals and universities first. Here are the batches mention in the Jablonowski and Hooker report:
Death: EL0140, EL9261, EL3248, EN9581, EJ1686
Serious AEs: EC4176, EK5730, EH9899, EJ1685
All AE’s: EK5730, EH9888, EK9231, EJ1685
All of these are the purple topped vials formulated in the PBS buffer.
2. FDA Report on Particulates
I have a report from the original EMA leak that most people have not read. This report was trying to get at the two issues that arose from the Process 2 vials which were not present in the Process 1 vials. (Note, the AMOUNT of residual DNA between Process 1 and Process 2 was not substantially different, it was the TYPE of DNA that changed; PCR generated vs plasmid DNA). But the issues that the regulators were concerned about were:
Particulates
Late Migrating Species (LMS) or what became known as Lipid Adducts, Lipid Impurities, and “stable folded RNA”
We will leave LMS and lipid adducts to Part 2.
Particulates
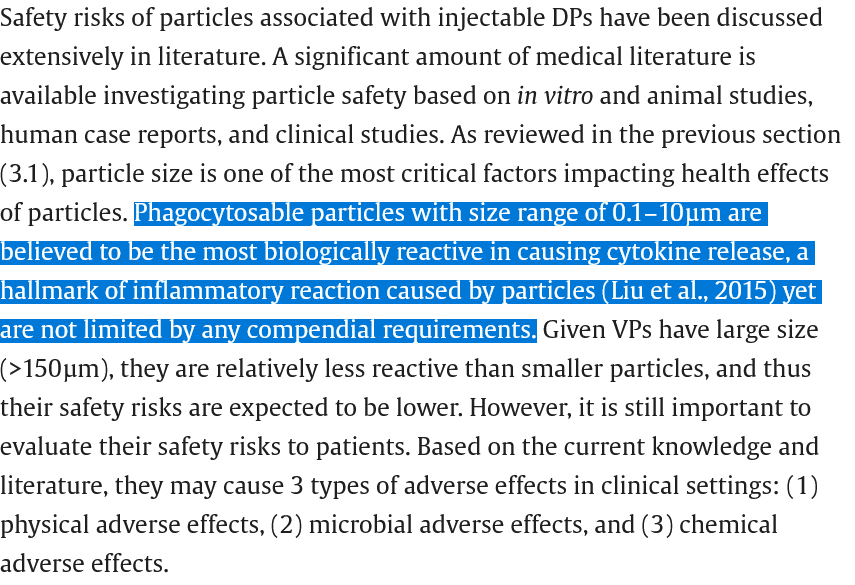
Let us look at particulates. These are a VERY BIG DEAL in sterile injectable products. My techs find particulates in sterile injectables from time to time and send them back. If they are visible, this is considered a manufacturing failure. Visible particles are those >150 microns or bigger. Here is a recent review which is unusual since the last one I found was about 10yrs ago.
My review on Particulates in Pfizer for more background.
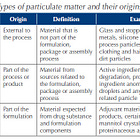
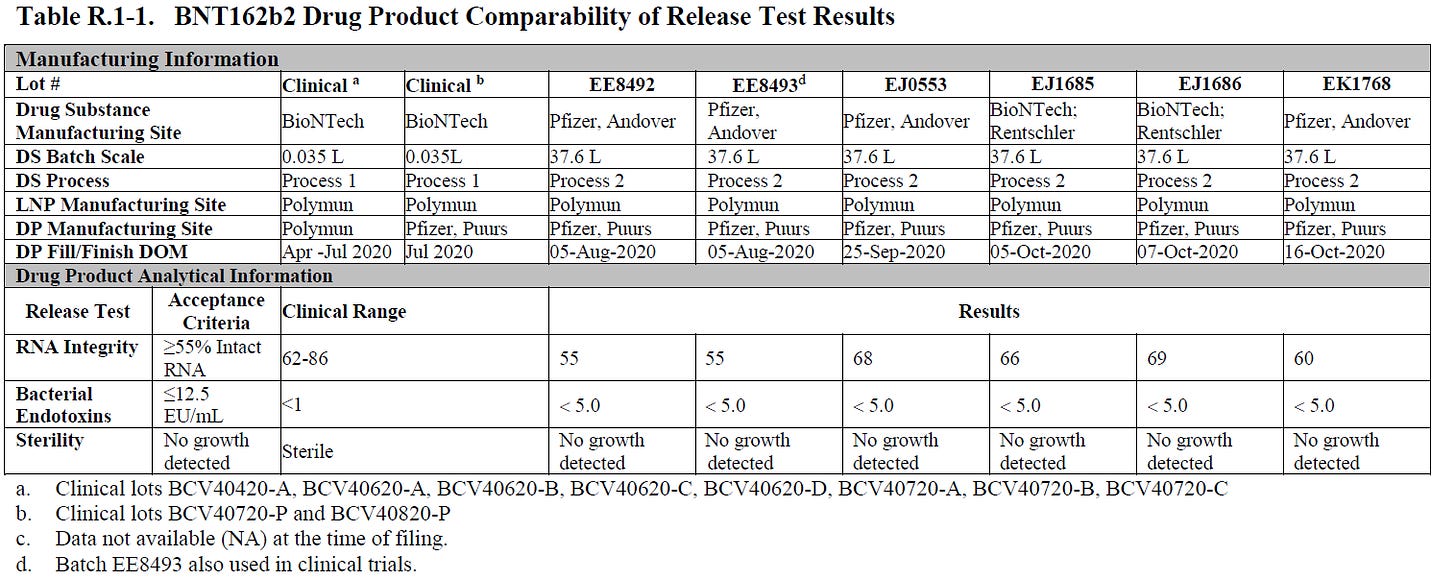
Here is a table comparing the Process 1 vials to the Process 2 vials. Note that Lots EJ1685 and EJ 1686 are some of the EARLIEST lots produced, manufactured Oct 5 and 7th respectively in 2020.
You can see here that Process 1 vials have NO visible particles, while Process 2 vials have visible particles as well as a number of subvisible particles. You have the change FREE from observable particles to ESSENTIALLY FREE from observable particles.
And according to the Jablonski paper above, the Lot EJ1646 was the most toxic as it appeared in total and serious adverse events and Lot EJ1645 appeared in the most deaths.
COULD IT BE PARTICULATES in the purple topped vials are one of the reasons for AE’s in the early lots?
RNA Integrity and Endotoxin
If everyone remembers, the EMA and other regulators were concerned about the drop in RNA integrity (intact modRNA construct) and potentially changes in endotoxin levels going from Process 1 to Process 2
As you can see here, measured endotoxin was higher than found in Process 1 vials, which could be another source of adverse events. Here, the RNA integrity is about 65-70% which affect EFFICACY primarily. Note that Lot EE8493 was used in the clinical trials. This is the lot used in those 250 patients as part of a clinical trial amendment which was never completed. Clinical Correlation of Process 2 Lots. But overall, since the change of RNA integrity affects efficacy this is unlikely the cause for the increase in toxicity of the early lots. Endotoxin remains an open question and I believe it may have played a role in the toxicity of the particulates.
3. Size of the LNPs and Adverse Events
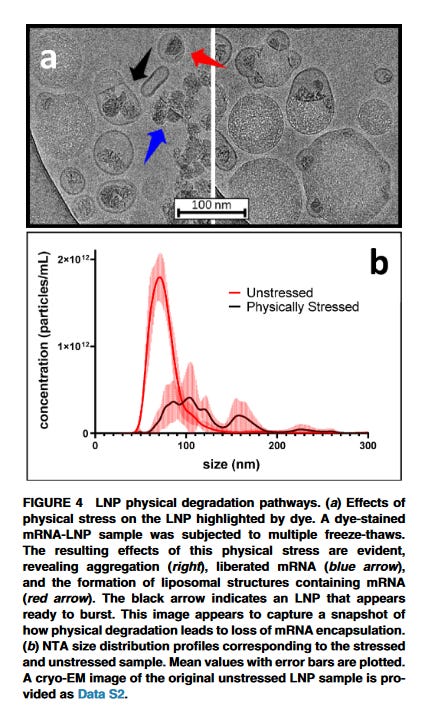
The poor formulation of these Process 2 lots in the PBS buffer results in instability of the LNPs, causing agglomeration and aggregation. These physiocochemical parameters are especially important in colloidal dispersions such as these vaccines. What does that look like in LNPs? This is from Mark Brader at Moderna who showed what happens to LNPs undergoing several freeze and thaw cycles. Note Moderna’s LNPs are in the Tris buffer which makes them more stable to freeze and thaw cycles compared to Pfizer’s purple topped vials.
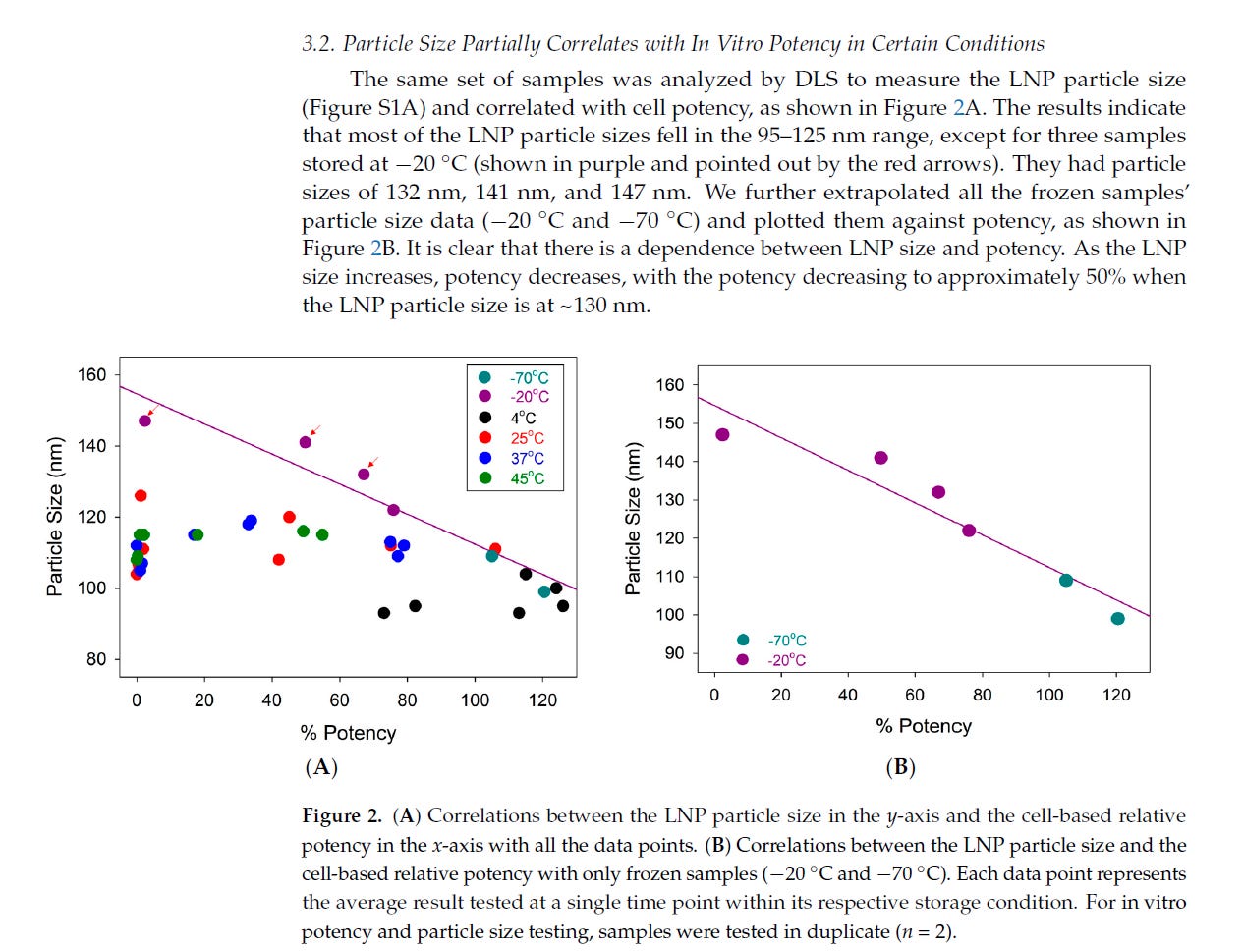
Some researchers have tried to look, at say particle size and in vitro expression, or the amount of protein produced. What they found is that LARGER particles are not producing as much protein as smaller ones.
Here is what I want to show you.
Thus, these lots with a number of particles that are large (ie the subvisible particles are 25 microns or 25,000 nm large) are not producing a lot of spike protein. The other smaller population of LNPs (ie 40-180nm) are the ones producing the spike protein. That is because these very large agglomerated particles are likely not being transfected. And what about those particles bigger than nanometer range (ie <200 nm) and those between 200 and 1000nm or even 10 microns which are not directly measured?
What toxicity do these large pegylated particles have?
Toxcicity of LNP Aggregates
There is very little data on the toxicity of LNP aggregates per se. Primarily as single LNPs. However, a review of nanoparticle technology noted:
OK, so this fits in with what we know. Aggregates can get too big to be endocytosed and to be transfected. However, some smaller aggregates do get transfected. Plus their physiocochemical properties change if they aggregate and their interactions with cells? Yikes. Do we know ANYTHING about LNP aggregates?
In general, particulates can cause lots of immunological effects just because of their SIZE.
You remember what size the subvisible particles they found in those early lots? The visible ones are >150 microns, then the medium ones are >25 microns or greater, and the smaller ones are 10 microns or bigger. But the size of the particles causing the MOST cytokine release are those 0.1-10 microns in size (100-1000nm) according to the article. AND THEY ARE NOT MEASURED. However, the large number of subvisible and visible particles may indicated there are MORE aggregates of LNPs in that 1-10micron range these authors are concerned about.
Relationship Between Size of Nanoparticles and Release of Cytokines
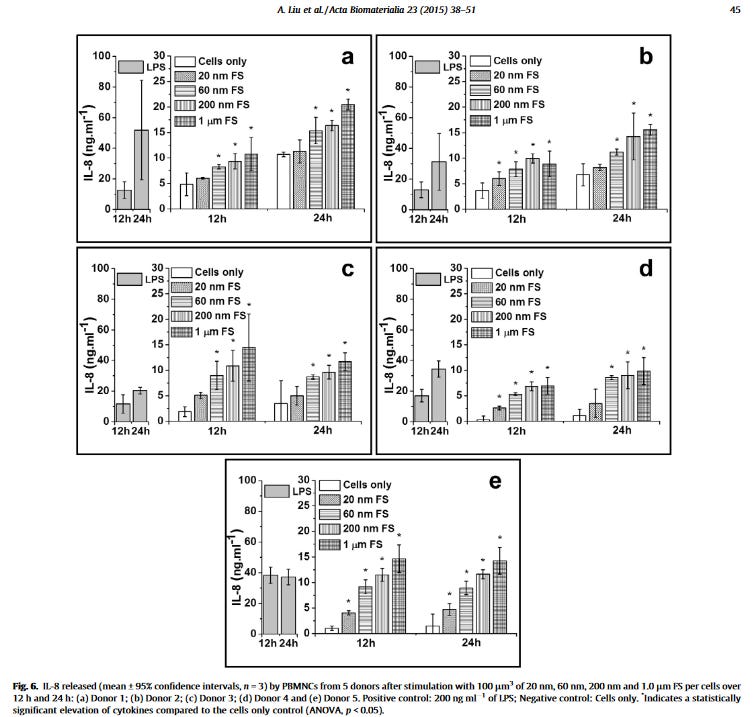
So in the linked article by Liu, they find cytokines, specifically IL-1beta, IL-6, IL-8 and TNF-alpha was released at greater amounts than even the control which was LPS (endotoxin). Think about that. For example, here is IL-8 in 5 patients.
They conclude that the results showed for the first time that nanometre-sized particles with a size range of <50 nm did not stimulate proinflammatory cytokine release including TNF-α, IL-1β, IL-6 and IL-8, from human peripheral blood mononuclear cells (PBMCs). HOWEVER, those about 1 micron size (1000nm) do. These are NOT lipid based nanoparticles but are polymers (ultrahigh molecular weight polyethylene) which are used in medical implants like knee replacements. But polymers act a lot like lipid nanoparticles.
Is this what is happening with the early shots? So if these aggregated LNPs (of about 1-10 microns) are transfected do they stimulate a lot of cytokine release? More than the 60-180nm range reported for the mRNA vaccines?
Some work on the parameters of nanoparticles and toxicity is reviewed in this excellent paper.
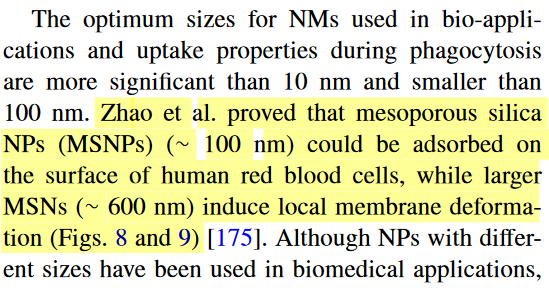
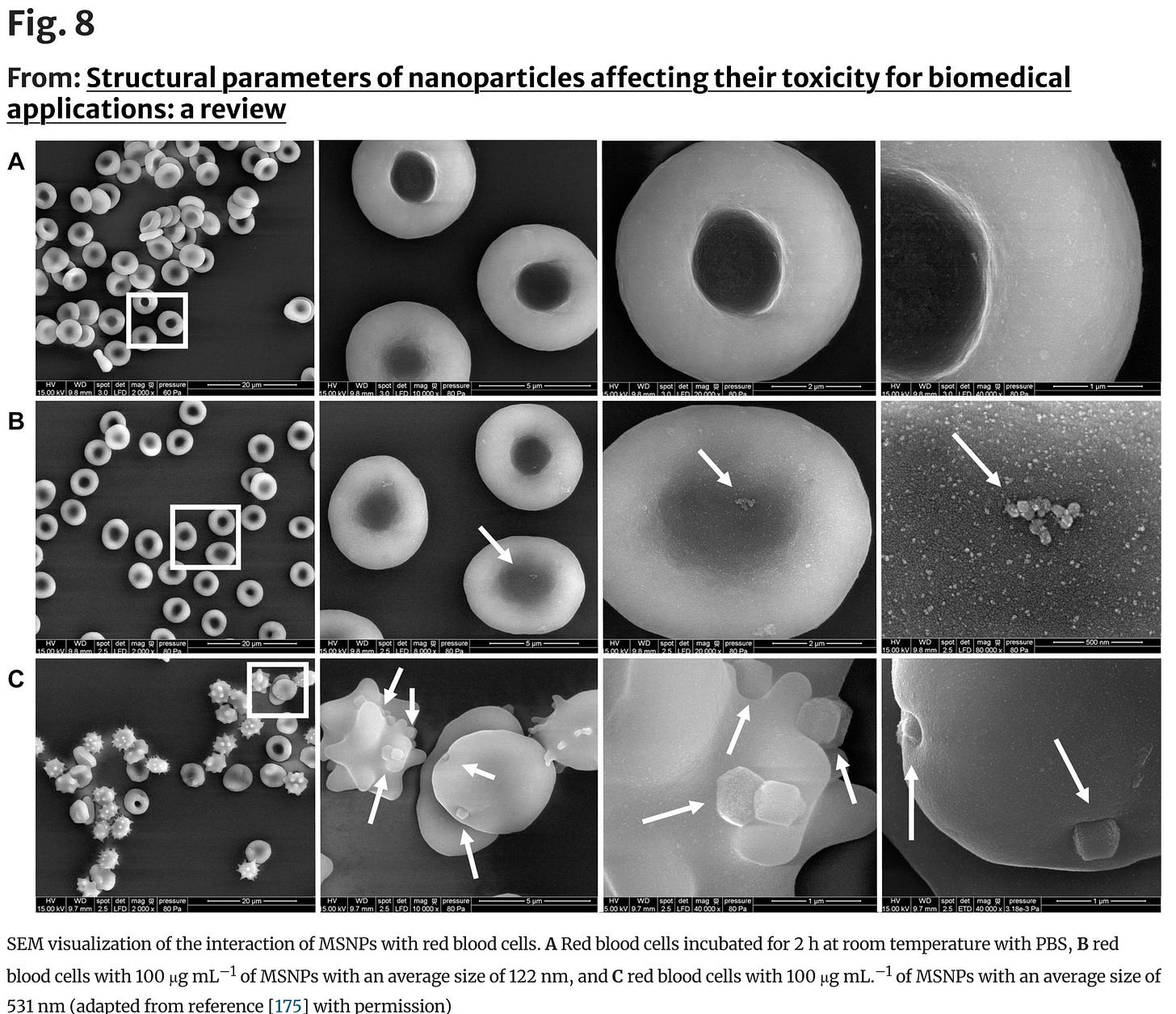
And here is an image of what aggregated nanoparticles do to the cell membrane by size (MSNP=mesoporous silica nanoparticles).
Oh I think
would be very interested in this. Look at what happens to the cell membrane.5. Dr McCullough Reviews Kounis Syndrome
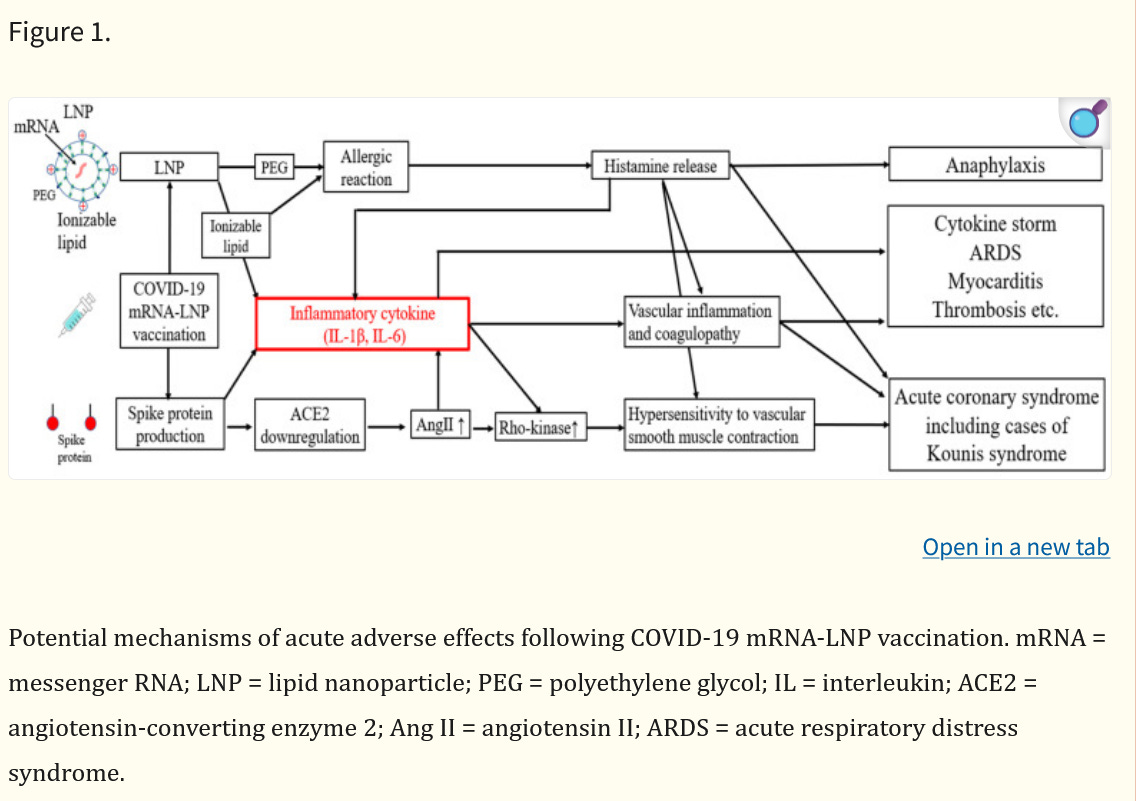
When Peter McCullough posted this substack article on Kounias syndrome a light bulb went off. He references the article by Awaya and here is a figure explaining Kounis syndrome and cytokine storm
Here you see both the ionizable lipid, the peg lipid and the spike protein contributing to cytokines such as IL-6, IL-1B and also IL-8 and TNF-alpha. These are the same cytokines released by the aggregated transfected nanoparticles in the above study by Liu. There is no doubt that various mechanisms of these vaccines release cytokines, but perhaps those early lots were more prone to adverse events such as the Kounis syndrome because of the known particulate problem? Anyone?
6. mRNA-LNPs are Unstable in Plasma and Serum
One other issue I have been thinking of for quite some time, is that particulates, are aggregates are unstable (by definition), and can break apart in serum and plasma. Maybe due to shearing forces? Venous blood is flowing at 5L/min when it enters the right atrium, though of course it is much less in capillaries etc. This article looks at this phenomenon.
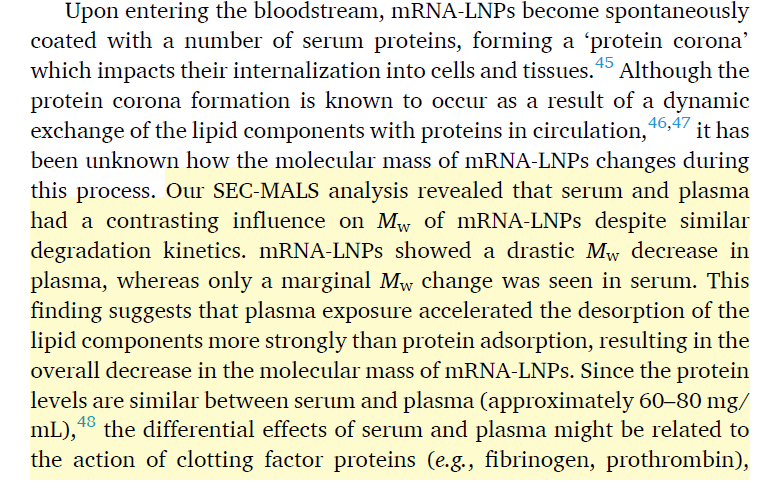
In this study they used size-exclusion chromatography to determine stability in blood and plasma by looking at the molecular weight of the mRNA-LNPs,
Uh oh. So what happens in plasma is that the PEG-lipid falls off (like it is supposed to), and then the ionized lipid, then the helper lipid leaving the cholesterol to encapsulate the mRNA. So does that mean the LNPS are BREAKING APART IN PLASMA? Oh and by the way lymph fluid is very similar in composition to plasma so this is what I expect is occurring even after IM administration and leaking into interstitial fluid and lymph.
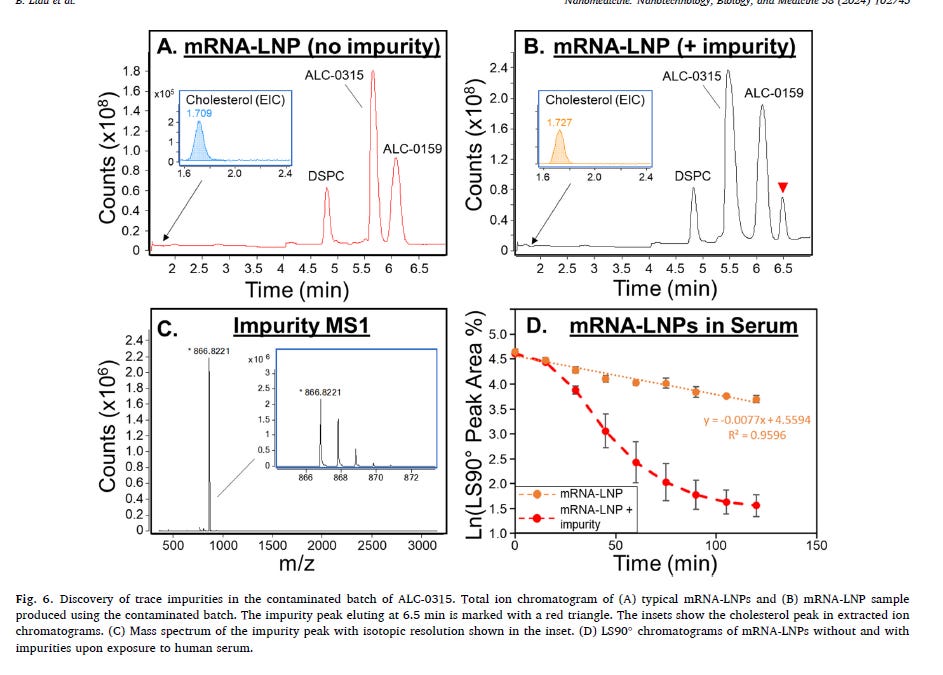
Oh, but what really surprised them is even a 1% in impurities which was found in the ionizable lipid ALC-0315 results in rapid degradation of the of the mRNA-lipids.
So impurities in the LNPs cause them to fall apart in the plasma, and pretty quickly at that (approx 2.5hrs)? See panel 5. Holy Toledo!. What impurities are these? Could it be the lipid adducts, greater in the purple top vials which have PBS buffer? I write about it here.
But right now I am interested in what kind of biological effects are there to broken up synthetic lipids and the modRNA in the blood or plasma? Does anyone know?
5. Does Bakos (with CARPA expert Szebeni) Have the Answer?
I believe this is an important paper that gives us clues to the adverse reactions, especially the allergic and anaphylaxis type effects which result in the Kounis syndrome as well as infusion related symptoms, known as CARPA (complement activation-reaction pseudoallergy).
In this study they incubate the Pfizer and Moderna vaccines in serum. For Pfizer they used lot ET7205 which was a purple topped vial (likely made in March or April 2021).
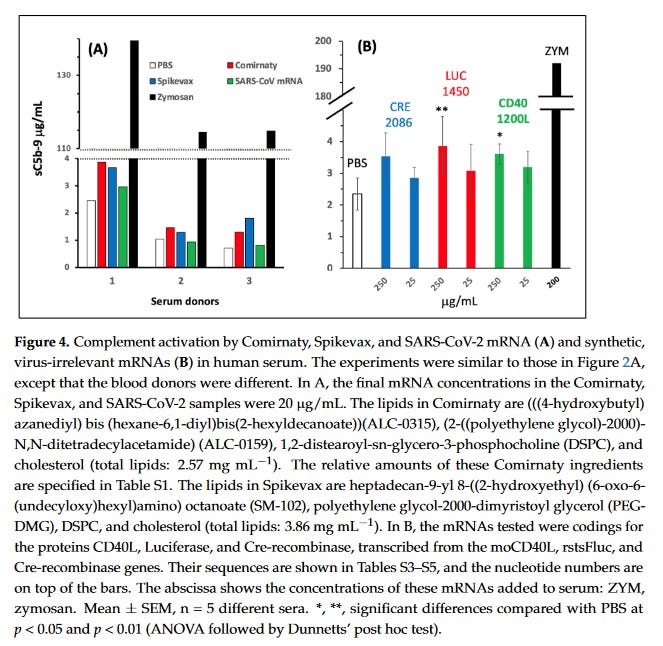
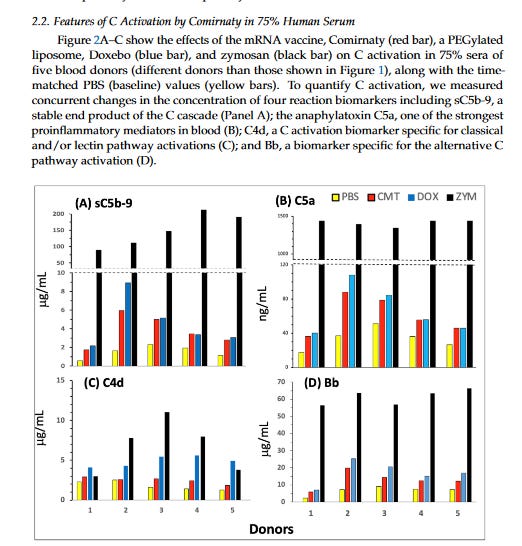
Firstly, lets look at the complement activation to naked modRNAs. Look primarily at Panel A. PBS is the control, Spikevax is the Moderna vaccine, Comirnaty is the Pfizer vaccine, the green is NAKED modRNA of SARS-CoV2, and ZYM is zymosan is a product derived from yeast cells commonly used in research to study inflammation and immune responses. You can see that naked modRNA is the least immunogenic in serum and doesn’t stimulate proteins in the complement system. In B they looked at other virus irrelevant naked RNA molecules which were still less immune stimulating than zymosan. Anyone who can expand on this is welcome to.
But when it comes to the PEGylated lipid or the ionizable lipids for both vaccines (i.e the whole LNP) then we see the effects in complement activation in human serum. What is very interesting is the pegylated doxorubicin, an anticancer agent, which has a black box warning for infusion reactions (CARPA) that often require pre-treatment.
And keep in mind the amount of lipid in Comirnaty was only 0.64mg/mL but for Doxo it was about 4mg/mL, yet the complement activation for C5a was comparable. There was no assessment for the risk of CARPA with these jabs, which should have been done given that these are pegylated nanoparticles like doxorubicin is. But because it was such a small dose and given IM, it was deemed uneccessary. I think this should be reassessed.
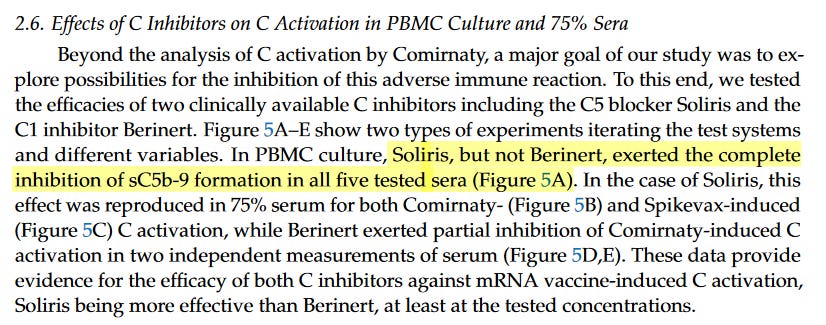
Furthermore, these researchers show that complement inhibitors can affect the cytokine production occurring with the lipids. Could this be helpful to the vaccine-injured?
So if the LNPs break apart in the serum, is the complement system activated by the pegylated and ionizable lipids? More than you would expect with intact LNPs? This is one of my hypothesis. Along with transfected aggregrated particles? Or that particulates of the LNPs set off the complement system and these allergic responses and cytokine release to a greater extent?
They conclude:
AHA! So the broken apart LNPs (like just ALC-0315) could activate the complement system like Cremophor EL. And THAT gives me the heebee jeebees because Cremophor EL brings me back to my undergraduate days using it as a vehicle for poorly water-soluble drugs. Shudder.
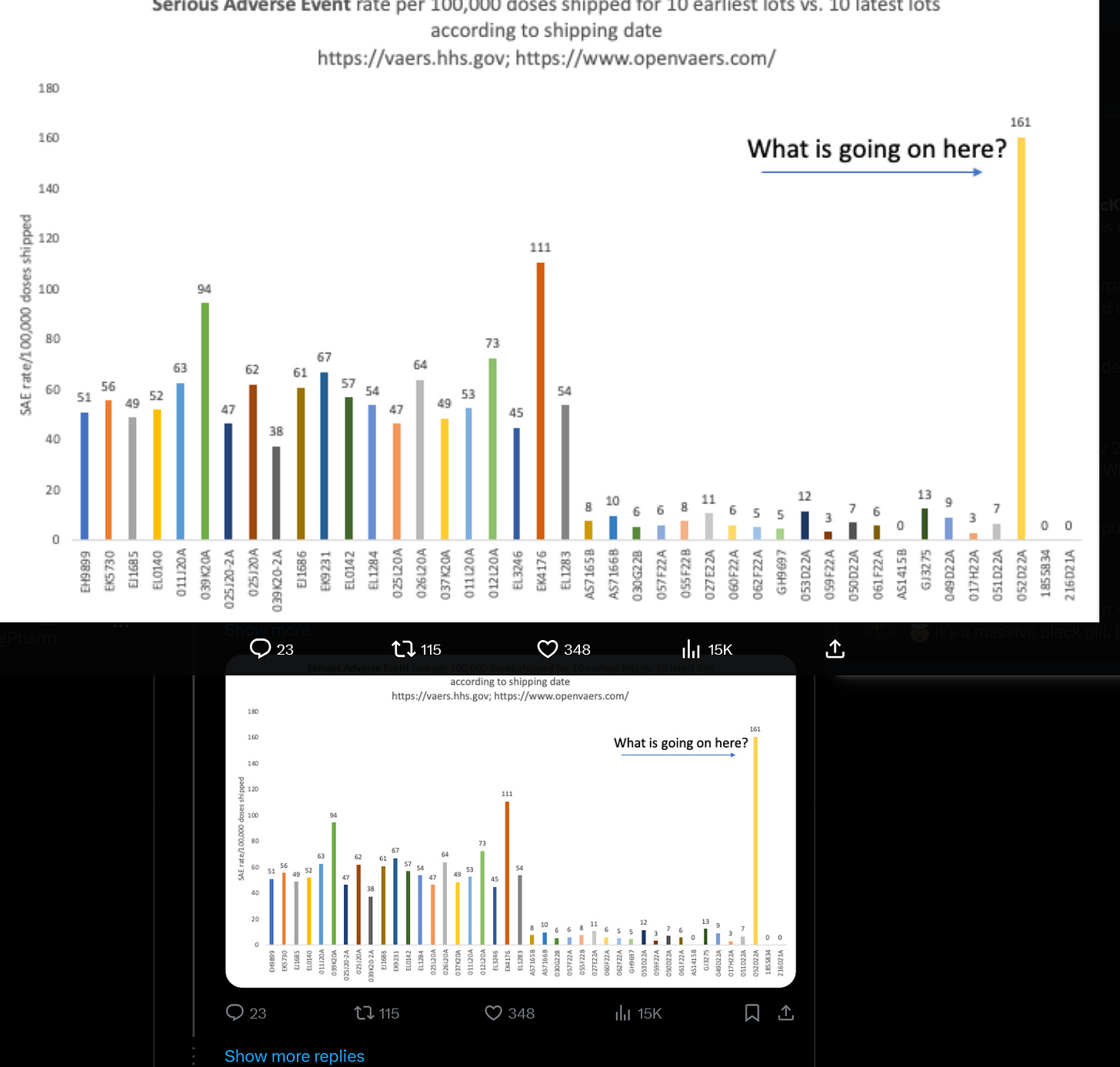
6. ’s Analysis Including Early Moderna Lots
When Jessica posted this graph to X, another lightbulb went off. Early lots of Moderna were more toxic than later lots? Well Moderna had lots of issues with their fill and finish and their lipids as well.
https://x.com/JesslovesMJK/status/1854399513437364434
See Moderna lot 052D22A? This is a Bivalent B.4/B.5 lot.
Here is an FDA letter that mentions these lots and approves their release. Why are these particular lots mentioned?
Well maybe because of this report FDA 483 from August 2022.
Do you see what they say about PARTICLES? Visible particles!! And the FDA released these batches! And in B, there is contamination with PROTEINS from a drug made just previously on the same filling line!!!
So the inspectors want to quarantine these batches, but the FDA releases them anyways. UNREAL.
And now Jessica shows one of these batches with lots of particulates have some of the highest reported AEs of Moderna lots. And how likely is it that the Moderna’s LNPs also formed aggregates in the early batches or when you have a new manufacturing process like with the Bivalents? And the SM-102 causes complement activation from broken apart LNPs like Pfizer does? I think that is possible and gives more support for my particle hypothesis. We need some more studies on this.
I write more on Moderna’s issues with particulates here. The stainless steel fiasco in Japan is also covered.
7. Are LNPs Toxic Inside and Outside the Cell?
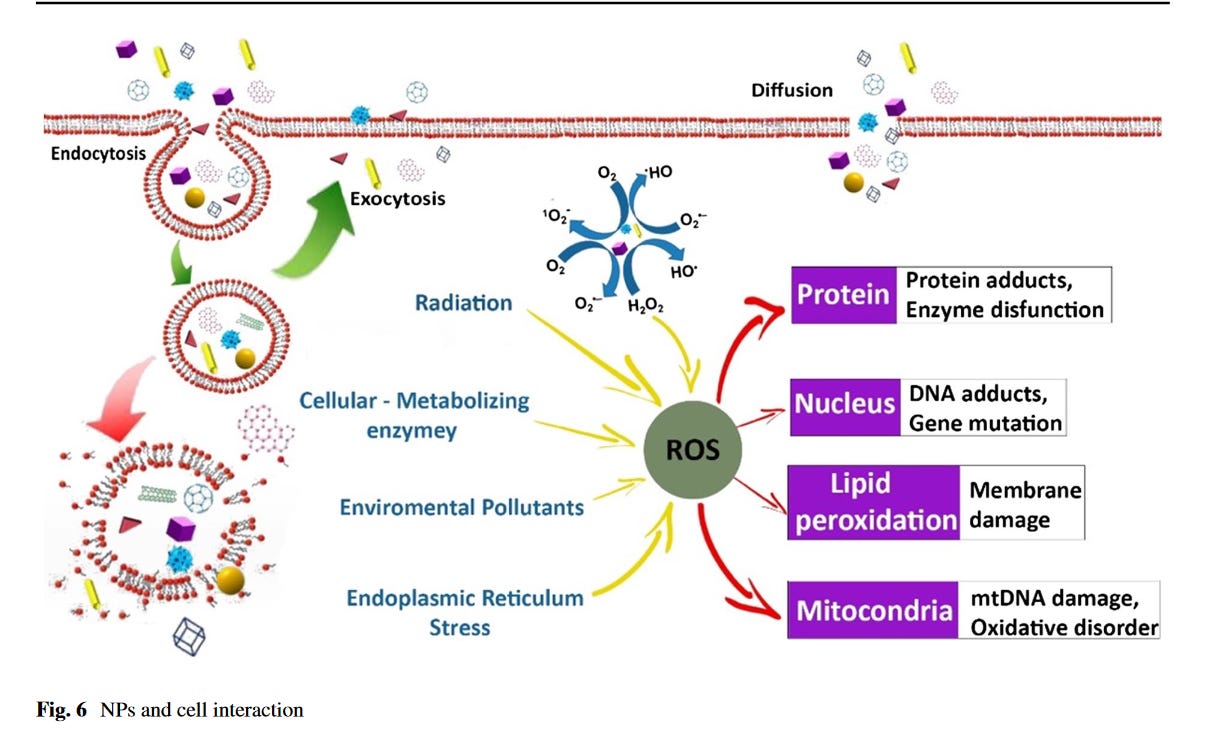
Inside the cell
I will finish with this image which I thinks summarizes what is happening with NP within the cell very well.
Outside the cell
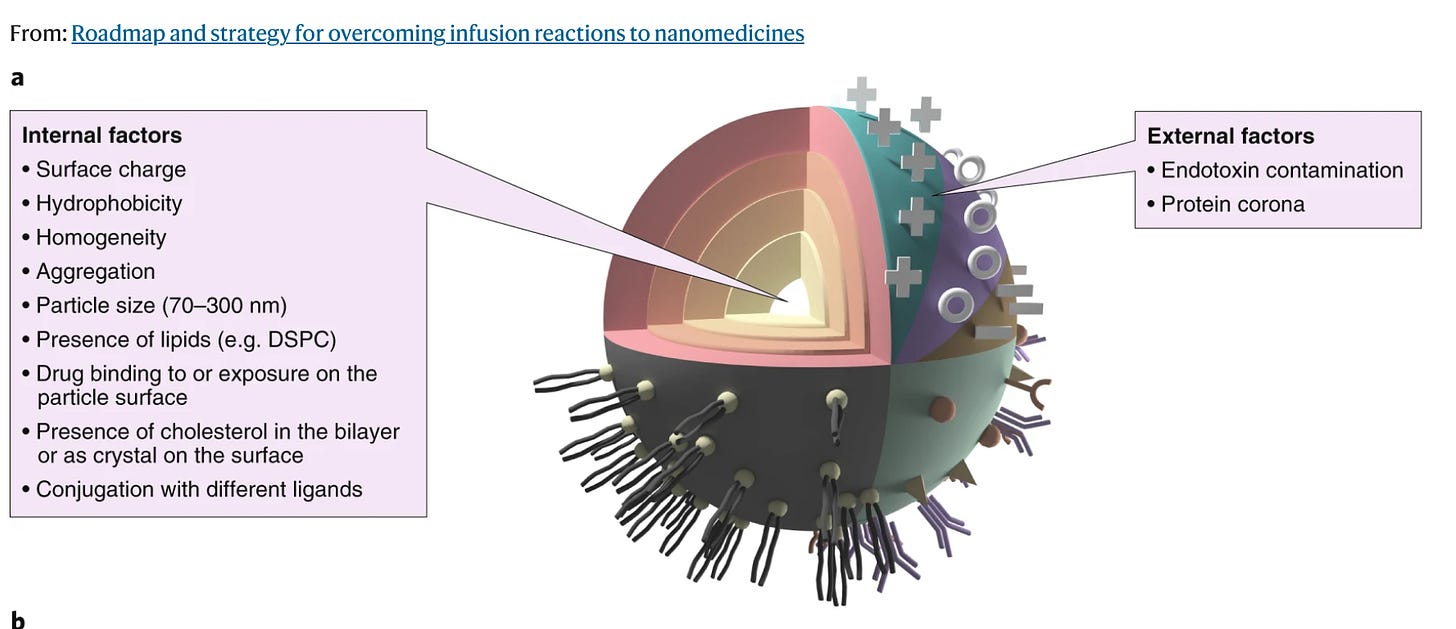
From: Roadmap and Strategy for Overcoming Infusion Reactions to Nanomedicines
Not a pretty picture
Summary
Particulates in sterile injectables are dangerous and are often overlooked as a source of adverse events
Early Process 2 lots had lots of issues with particulates, likely due to scale up issues and unfamiliarity with the microfluidic process in making the LNPs plus Pfizer had instability from using the PBS buffer.
LNP particulates maybe especially immunogenic but not because of the mRNA
larger aggregated LNPs can be transfected causing significant cytokine release compared to smaller LNPs
Very large visible particulates may have other independent AEs
Early lots had issues with stability resulting in aggregates and agglomeration; this in turn made them unstable in the blood and many were likely disintegrating after being injected with unknown effects. Known impurities made this worse.
The LNPs in serum can set off the complement system and cytokine release causing anaphylactic like reactions and possibly contribute to the Kounis syndrome. This may be more likely with aggregates but more work is required. Also the individual ionizable lipids alone or as aggregates could also cause complement activation.
The FDA released Moderna lots with visible black particles in it, contrary to the recommendations of its own analysts.
I am not sure that the production of spike protein, at least in the first few days contributes to the anaphylaxis/allergy or Kounis syndrome and AEs. I believe that particulates and the unstable LNPs and impurities play much more of a role.
What does everyone think? Am I off my rocker? I don’t think these mechanisms explain the ongoing cytokine storm vaccine injured patients are experiencing from the early lots UNLESS there is some metabolism or other issue at play.
Thank you for reading and please pray the rosary. These injections need to be stopped.









I am happy that all these people are discovering the batch variability problem. However, none of them seem to have courtesy to mention that I was the first person in the world who published on this, in October of 2021, including the fact that earlier batches were super toxic. And none of them refer to Craig's website howbad.info, and how we tried to get everyone's attention to this for 2+ years, and nobody would listen. As if that never happened! And no, it's not the "particulates". These were smaller batches, and they were used up almost 100%, and they were more "to spec" in terms of RNA integrity and other ingredients vs. later huge batches that are combined from multiple production runs (as the shipment data shows). The uptake of the shots declined while the scale of production increased. This simply proves that the deaths and injuries are definitely caused by the shots, the usage is directly proportional to death. But also variability at larger scale is larger.
I, too, am a washed-up pharmacist. I’ve had nothing better to do for 5 years than read over 3,000 articles on the subject of the virus and the vaccines. Just when I think I can’t be more horrified by this whole fiasco, along comes yet another brilliant scientist with a new catastrophe! Visible particles? This is Intern Pharmacist 101– No Visible Particles Ever, Not One. What in the world…