A review of Brook et al on adjuvantation and control of spike protein expression
what it tells us and what it doesn't
I’ve just come back from a refreshing week in northern Michigan visiting my in-laws, just chilling, boating and getting sun. It was very hot this week, especially for the upper peninsula.
I saw in passing 2 articles that were of great interest to me and illustrates why we must understand the pharmacology and pharmacokinetics of these modRNA products well. These issues are the “pro” part of these jabs and must be understood in order to parse what you are being told and what is NOT being studied. I will briefly examine the Adjuvant Paper first which was brought to my attention by the wonderful and insightful
. Thank you!Adjuvant Paper
Here is the first paper I want to quickly review, found here
Most of the authors are from Precision Vaccines Program, Department of Pediatrics, Boston Children’s Hospital (don’t you love the word, “precision?”) or from a company called Combined Therapeutics Incorporated also in Boston.
Here is the summary
Adjuvantation of the BNT162b2 SARS-CoV-2 mRNA vaccine with an mRNA encoding IL-12p70 amplified humoral and cellular immune responses, including in aged mice, and enabled a reduced dose of vaccine mRNA needed to achieve the same antibody response seen without adjuvantation. Moreover, limiting expression of IL-12p70 or spike antigen to muscle tissue, through the use of a multiorgan protection (MOP) sequence, maintained the benefit of adjuvantation with the potential advantage of avoiding side effects caused by systemic transcript expression. These data support further development of this mRNA-based adjuvant and of MOP platforms.
Adjuvantation
Here they are ADDING another modRNA construct that encodes a cytokine IL-12p70, in this case specific for a mouse. So what you have here is similar to the bivalent vaccines. How well do BOTH modRNA get translated? Did they look at that? Is it possible that chimeric proteins are made? So many questions to be answered.
Now if you think this is a new concept, be advised that this was already being considered in mid 2022 or earlier and documented in the WHO 2022 Evaluation of the quality, safety and efficacy of messenger RNA vaccines for the prevention of infectious diseases: regulatory considerations which you can read HERE
The rationale for the selection of the target antigen(s) or parts thereof and of any proteins (for example, cytokines) that are encoded, as well as their contribution to the proposed mode- or mechanism-of-action (proposed protective process) of the vaccine, should be clearly described.
Additional immunomodulators or adjuvants: the mRNA might also encode specific immunomodulatory molecules such as cytokines. Furthermore, a separate adjuvant or immunomodulatory (stimulatory or suppressive) compound not encoded in the mRNA might be added to the formulation or as part of the LNP. As a general principle regarding vaccines formulated with adjuvants, a demonstration of the contribution of such an addition to vaccine immunogenicity should be provided. Quality aspects of the separate adjuvant, if included, should also be addressed and described.
So this CYTOKINE would be considered an ADJUVANT under vaccine guidance and have limited study as to its toxicology, maybe in vitro testing of possible carcinogenicity or genotoxicity, and some preliminary biodistribution as shown here. That is it. Nothing long term, no secondary pharmacology, persistence, elimination etc. What could go wrong? What are it’s off target effects?
Multi-Organ “Protection”
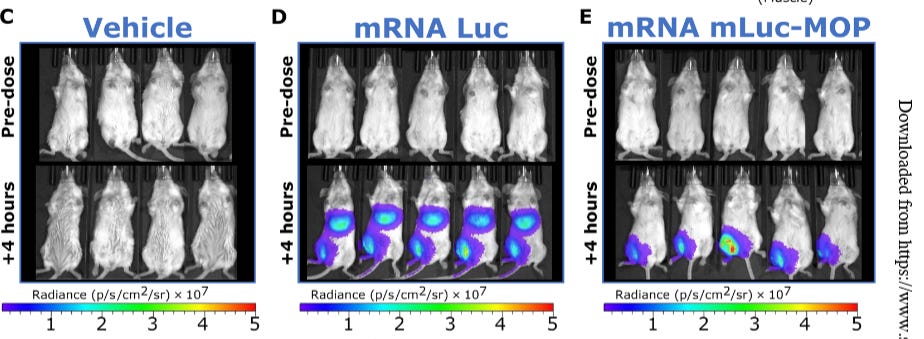
Here is a great figure summing up the experiment with the MOP sequences.
Here if you look at regular modRNA and this new MOP modRNA you would believe that the modRNA stays mostly in the muscle. But that is not true.
What this is showing is that the TRANSLATION of the modRNA is limited to the muscle by using this multi-organ protection which is a miRNA in the 3’UTR of the modRNA construct for the CYTOKINE. This does NOT mean the modRNA stays in the muscle. It is still distributed to liver, kidney and heart. It is just that the mRNA is NOT translated in those tissue. So what happens to this modRNA? Does it just sit there in the endosomes/lysomes? How does it get metabolized or cleared? Are there ramifications of the MOP sequences?
MOP leveraged gene-silencing miRNAs to enable tissue-specific control of transcript translation
So this modRNA are GENE SILENCING in tissues other than muscle tissue. Is anyone going to look at this? Not if vaccine guidelines are used because they don’t consider the ‘pro’ jab pharmacological action of this product.
And what happens to the modRNA for BNT162b2? I looked at the methods which you can download HERE
mRNA encoding full-length spike protein coding sequence (CTX-382), used in Fig. 4 and fig. S9, was designed with K986P and K987P substitutions to produce a prefusion stabilized SARS-CoV-2 protein, and codon-optimized using the Gensmart Codon Optimization Tool (Genscript). Transcript uridines were replaced by pseudouridines. This construct included the CTX MOP sequences within the 3’UTR.
Ok so the modRNA for the actual spike protein ALSO has the gene silencing MOP sequences in the 3’UTR. PLUS pseudouridine.
Lovely.
The Steps in the Pharmacological Action of modRNA
When I read the abstract, my first question was, what about the non-translated modRNA? IMHO it is very important to understand these steps in order to put all these new approaches to modRNA vaccination which supposedly addresses the drawbacks of the first generation vaccines. As you can see, they KNEW about these drawbacks very early and were already working on “fixes” as evidenced by the WHO guidelines.
So to reiterate, here is the pharmacological action
administration
protein corona formation
biodistribution
tranfection
endosomal escape (the rate limiting step)
release of modRNA
translation in ribosomes for protein expression
Reading even just the abstract made me realize they are really only addressing step 7.
Oh and for you keeners who actually read the paper….this figure
… only shows where protein expression occurs (using luciferase which is not sensitive enough anyways and not accepted by the regulators, but it looks impressive) and NOT WHERE THE LNPs bio-distributes. For that you would need to radio-label the cholesterol in the LNP.
This is another sounds good on paper idea, but is just as problematic as the original modRNA. In addition, this paper admits the following
the modRNA ‘vaccines’ need an adjuvant to have long lasting immunity
so I guess the dsRNA is not working out too well Moderna? and neither is the dsDNA for Pfizer?
another track is using the LNPs with a cytokine imbedded in the outer surface as an adjuvant, or endotoxin. I expect a paper on this, or maybe there is one already. Anyone?
the modRNA ‘vaccines’ have a wide bio-distribution which is detrimental and a cause of adverse effects.
otherwise why try minimizing it with GENE SILENCING METHODS. Holy Toledo what are they smoking
the modRNA ‘vaccines’ used too high a “dose”
only the amount of modRNA in the formulation does not consistently translate to MORE spike protein. That is dependent primarily on the endosomal escape which is dependent on various factors, but primarily how well the ionizable lipids pronate and a whole bunch of other stuff I’m trying to work out.
and with usual vaccinology, decrease the antigen amount and use a powerful adjuvant. Only with modRNA vaccines it is a biologic making a biologic (ie the “pro” part of this product) and there is no lovely sigmoid curve on mRNA amount and antigen production with which to base a dose on (that I know of that is consistent and reproducible). Anyone?
Next I’ll review that new so called biodistribution paper which has a lot of questionable conclusions imho.
Thanks for ready and of course, pray the rosary.






Thank you for this excellent analysis. I had actually wanted to discuss this paper also but was side-tracked by other things. Glad you exposed the many issues! I definitely want to re-iterate - you already said it so well. But the issue is that you have these synthetic things STILL go everywhere, and with these, you WILL have some effect. God knows what. In THEORY only, they will only get translated in muscle tissue. I don't think the experiments fully prove this. Seems to me the tissues and organs analyzed were limited. Did you see anywhere for how long they assessed the whole thing? I don't recall but I suspect they could not really analyze the long-term effects of the synthetic culprits. AND: it still doesn't prove it's limited to the injection site. It may wreak havoc on muscle tissue all over the place! Thanks again for exposing so many issues!
Good suggestion....the Rosary. Best Regards