I apologize for not writing recently. It has been very busy and I have had to deal with some serious issues with my very elderly parents. So this will be a short one, but I hope interesting.
As you know BioNTech started researching the manufacturing of the jabs in January (maybe before) but the first biodistribution study started in Jan 2020. Here I want to concentrate on the mRNA.
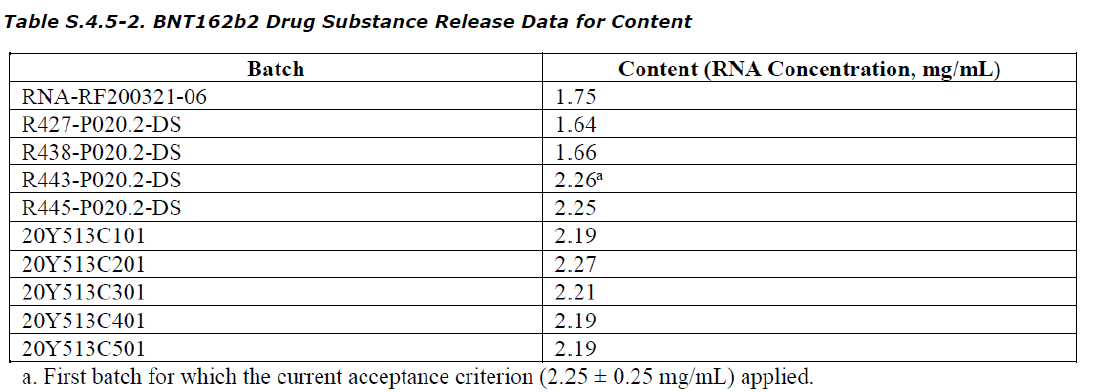
Lot RNA-RF200321-6
The very first lot of the modRNA BNT162b2 was made on March 27, 2020 as shown below. It was called an engineering lot, and it was a non-clinical and toxicology lot.
Here are all the lots of BNT162b2 that were manufactured prior to authorization of the vaccine. Notice all the Process 1 lots were made by BioNTech, and the first lots of Process 2 was made by Pfizer using the infamous plasmid. These Pfizer lots are the Process Performance Lots (ie the lots all subsequents lots are to be compared). Also note WHERE the Process 1 lots were made. Our special lot, RNA-RF200321-06 was made in a different lab than the other lots used in the clinical trials made by BioNTech. BioNTech RNA Pharmaceuicals but all the other Process 1 lots were made in BioNTech Innovative Manufacturing. Does this signify 2 different companies?
Quality Attributes of Lot RNA-RF200321-6
RNA Content
Lets look at the characteristics of this mRNA lot. Note this lot number is for the mRNA itself. There are other numbers associated with the LNPs and yet another number associated with the final drug product.
Here we can see the RNA concentration per lot. Our lot has 1.75 mg/mL. Now this is lower than the commercial lots we are using currently which are 2.25mg/ml +/- 0.25mg/mL. At this point I don’t think is unusual, just BioNTech determining the best “dose.”
Remember that any lot beginning with 20Y513 is a process 2 lot made by Pfizer in 2020
RNA Integrity (ie % intact RNA)
Here, we see this special lot conforms to the other Process 1 lots. Please don’t ask me about the reprocessing method yet. That is hard to figure out even with the detail in the EMA leak/hack. Anyone else know, please chime in. For now, we can see that there is nothing different regarding RNA integrity and the proportion of intact RNA when comparable to the other Process 1 lots.
Residual DNA
Ok, here is where it gets very interesting. This lot had a TON of residual DNA. It is a fallacy that Process 1 lots did not have DNA in it. It did. It just wasn’t plasmid DNA. It was amplified synthetically made DNA.
But look at the amount. 815.3 ng DNA per mg RNA. BioNTech helpfully adds that Oh well, our DNase didn’t work. Even with this very first lot, they had problems with their DNase enzyme. Why is that? However, it was March 2020. Was there not time to make another engineering lot that had less DNA in it? Why was it cleared to be used in studies? This will become important later.
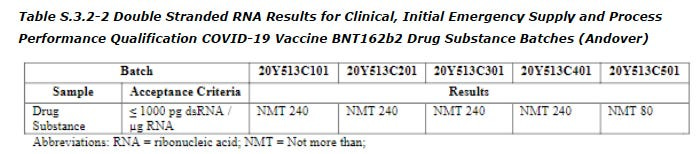
Residual dsRNA
dsRNA is a byproduct of the in vitro transcription of the DNA template to mRNA. I have written a lot about dsRNA which is not talked about enough. This impurity is likely the cause of myocarditis among other issues. Check out my previous substack on this.
dsRNA, Residual DNA and a BioNTech Patent
·BioNTech Patent found by Patent_Sun on X I don’t know who Patent_Sun is personally but he he is a thorough and very bright Japanese attorney who looks at and interprets patents and here he found a doozy.BioNTech Patent. He is well worth following on X.
However, the level of residual dsRNA for our special lot WAS NOT REPORTED. The only dsRNA reported are for the Process 2 lots reproduced below.
Furthermore, other attributes like the 5’ cap, the poly A tail etc were also not reported in the EMA leak/hack. But if the manufacturing of this special lot was like all the other lots made by Pfizer, then there is likely an inverse relationship between the amount of DNA and dsRNA. More residual DNA means less dsRNA, based on the linked entry and on the BioNTech patent. So it is likely there is negligible levels of dsRNA in this special lot.
Where was this special lot used?
This special engineered lot with a LARGE amount of DNA but I believe almost no dsRNA was used in the following studies.
R-20-0085 Immunogenicity in mice
performed March 31st to September 17, 2020 (only days after it is made)
VR-VTR 10741 Immunogenicity, transmission, etc in non-human primates
performed April 4-Aug 18, 2020
R-20-0112 Immunogenicity in mice
June 22-Aug 14, 2022
R-20-0211 in vitro expression, western blots and potency testing
June 22-Aug 14, 2020
20GR142 toxicity study in rats
June 23-Aug 13, 2020
20256434 the DART study in rats (development and reproducibility)
Sept 27-Dec 10, 2020
All of these studies can be found at Public Health and Medical Professionals for Transparency link which is Aaron Siri’s site. Just paste the study number in the search field and the study will come up.
Also notable is that this lot was used as late as September for the DART study. There was plenty of time to make another Process 1 engineering lot I think.
So WHY?
Here are some possible explanations
it was too expensive and difficult to make another engineering lot so they went with this one
this lot likely had almost no dsRNA so there would not be any innate immunity complications and toxicity to explain away. No myocarditis etc See my very first substack on this DNA, dsRNA, myocarditis etc. So they used it in all the toxicity and immunogenicity trials in mice, rats and monkeys. Thoughts?
Perhaps this was not a codon-optimized BNT162b2 lot with no dsRNA, so no frameshift proteins either? You can’t tell from the EMA hack if this special lot was codon-optimized or not. Just another speculation.
It had no plasmid DNA but still quite a bit of DNA. Was the DNA used as an adjuvant instead of the dsRNA? Is it “slower acting?” less acute effects than dsRNA? Comments anyone? This is stretching the limits of my knowledge.
So many questions. Anyone have any other ideas? I am all ears.
Thanks for reading
Oh and pray the rosary








> At this point I don’t think is unusual, just BioNTech determining the best “dose.”
After reading a lot of detailed articles like yours, I am always left with a question.
We deal here with biological “manufacturing”. Which means that some stuff is put into huge vessels and left there to react, with the objective of generating some other stuff. However, all key ingredients are submicroscopic (i.e. uncountable) and are of biological nature, i.e. uncontrollable in bulk. By common sense analogy, when you “boil” this bio-soup, it may have some pre-defined theoretical generic taste and properties, but - if you take it to a lab - every single spoonful will be different, with different properties. Hence, the “quantity” of the ingredients in vials vary immensely. To simplify, how can you “force” and “mandate” xxx trillions of particle-size live bio-organisms to interact to produce (say) 27 resulting products and stop at that point? Submicroscopic - which means that we really don’t know what is going on there. We can’t measure it, we can’t monitor it in real time, and even if, we can’t stop it right here because of momentum and variability of processes.
Unlike with chemicals - you take 20 pounds of powder A, mix it with 30 pounds of powder B, add 5 pounds of powder C, mix it all together and (since the ingredients do not interact), you can easily create 55 pounds of perfectly uniform final mixture.
Hence: the concept of “determining” the best dose seems to me absurd. One, each dose will be invariably different. Two, none of these were (or could ever be) truly tested on live organisms (because of the variability of these organisms and their snapshot conditions).
How do you command 200 liters of live organisms continuously interacting with each other when it is technically impossible to know what is in the boiling installation at any given point in time?
Even if there are some theoretical ways to force the end of the interaction (temperature change, pressure change, etc.), the very mechanism of halting the process will always be extended over time (even if in milliseconds) - so the final composition in a 200-l container will always be varied and impossible to identify in quality and quantity terms.
What am I missing?
dsRNA can be cut by dicer into microRNA. This lot thus avoids an RNAi signal.