CARPA , ISRR or Anaphylaxis?
Is CARPA Being Misclassified?
New Name, New Look, New Logo
On the recommendations of my colleagues I have changed the name from WashedUp Pharmacist to OffScript Pharmacist. I still feel washed up from time to time from my previous life, due to the Covid years, and some very tough professional times I went through. However, I am hoping that my new role in researching and assessing the mRNA vaccines helps bring justice to my sister and to all of us. But I am still Off Script, lol.
BTW, the logo is supposed to be an LNP coming out of the capsule. Well, I tried.
Introduction
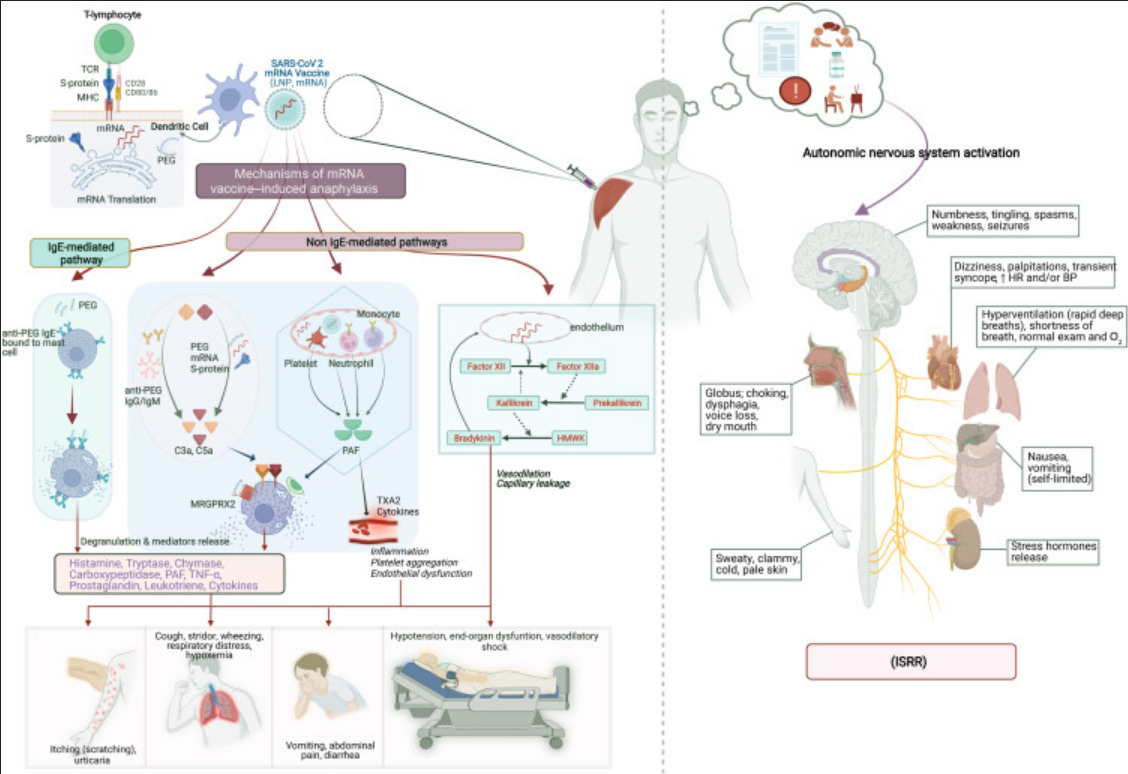
Since the global rollout of mRNA–LNP vaccines against COVID-19, there has been lots of reports of immediate post-injection adverse events. These can range from flushing and palpitations to syncope and chest tightness. While some are diagnosed as IgE-mediated anaphylaxis, there is another class of responses, that the WHO Brighton Collaboration call Immunization Stress–Related Responses (ISRR). However, I believe that complement-activating properties (CARPA) from the LNPs specifically the pegylated lipids are a better more biologically grounded explanation for at least a subset of these reactions. I also believe that CARPA is under-recognized in pharmacovigilance.
What is CARPA?
First, review my previous post on CARPA to understand why these LNPs are very likely to cause this reaction. See
CARPA
I think I have been talking about CARPA since 2021. It is HARDLY talked about in the literature, among most MFM doctors and I really do not know why. IMHO, the fact that CARPA occurs, was predicted, and was minimized is another huge red flag regarding these so called vaccines.
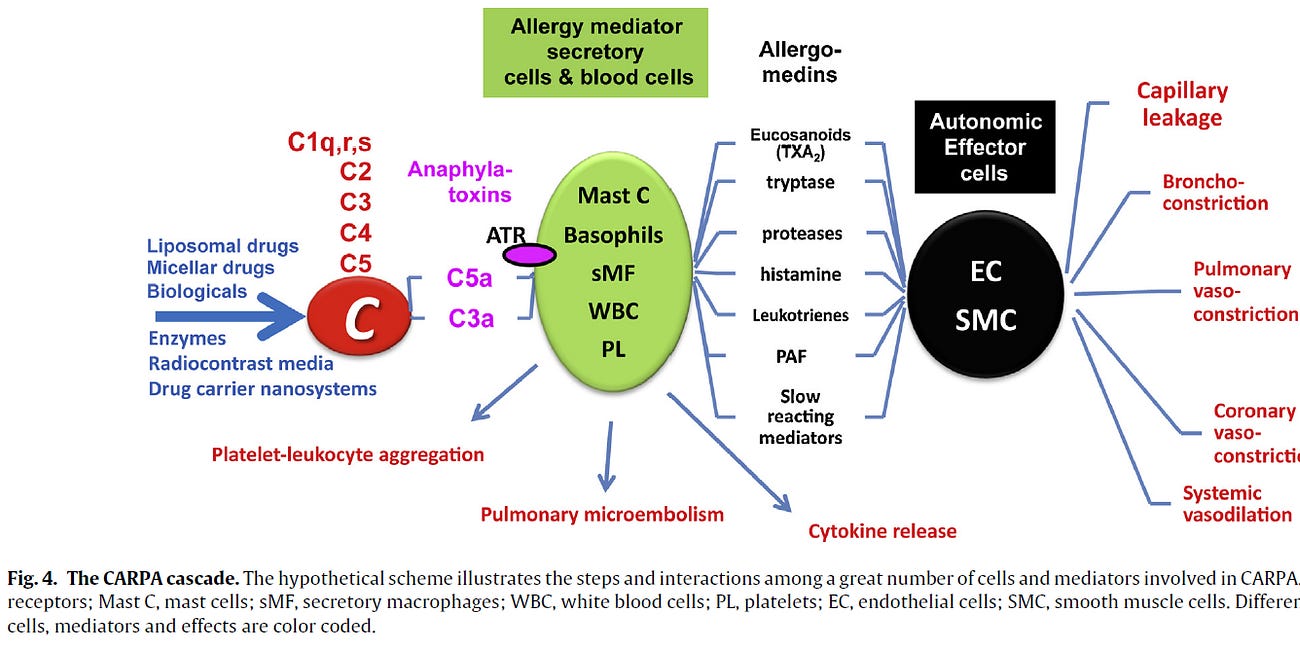
CARPA is a well-established, innate immune reaction triggered by complement activation especially via IgM or IgG antibodies binding to surface-modified nanoparticles such as PEGylated liposomes. This leads to the generation of C3a and C5a anaphylatoxins, which in turn activate mast cells, basophils, and pulmonary intravascular macrophages (PIMs), resulting in cardiopulmonary distress.
CARPA has been documented extensively in animal models and human trials involving:
Liposomal doxorubicin (Doxil)
PEGylated nanomedicines
LNP–mRNA vaccines
This reaction is not infrequent in outpatient oncology where liposomal doxorubicin is used, or for us hospital pharmacists working with antibiotics. CARPA like reactions to the antibiotic vancomycin we call the redman syndrome, or anaphylactoid reactions.
A Few Important Clinical Points Regarding CARPA
Mechanism Summary
Pre-existing or induced anti-PEG IgM/IgG binds LNP surfaces → triggers complement activation → generates C3a/C5a anaphylatoxins → leads to mast cell and immune cell activation.
This results in symptoms like
tachycardia,
chest pressure or back pain
hypotension
rash
Dose Response
Something important to know about CARPA is that it is idiosyncratic, not strictly cumulative with respect to dose (which is different usually from anaphylaxis which often is dependent on the amount of toxin the patient is exposed to).
First Dose
You do not need a previous exposure or sensitization like you do with anaphylaxis. That is because there is natural IgM and IgG antibodies already present in people. Many people have pre-existing anti-PEG antibodies from exposures to cosmetics, drugs, or food products, which can activate complement on the first exposure to a different drug, ie the LNPs. And I believe that is why females tend to react more to the mRNA vaccines since they handle food and cosmetics generally more than men.
Subsequent Doses
This is what is often not well understood with CARPA. The risk of CARPA may increase with repeated exposure if the anti-PEG antibodies are boosted (ahem…), immune memory activation, or if there are individual changes to the patients’ subsceptibilities. HOWEVER, risk is NOT LINEAR. Some patients get no reaction to the first dose, then will to the second dose, then increasing severity of reaction with more doses. Other patients, get tachyphylaxis and do not get any further CARPA reactions. But CARPA is unpredictable so we would assume once a patient had a CARPA reaction they would get another one, often more severe on subsequent doses.
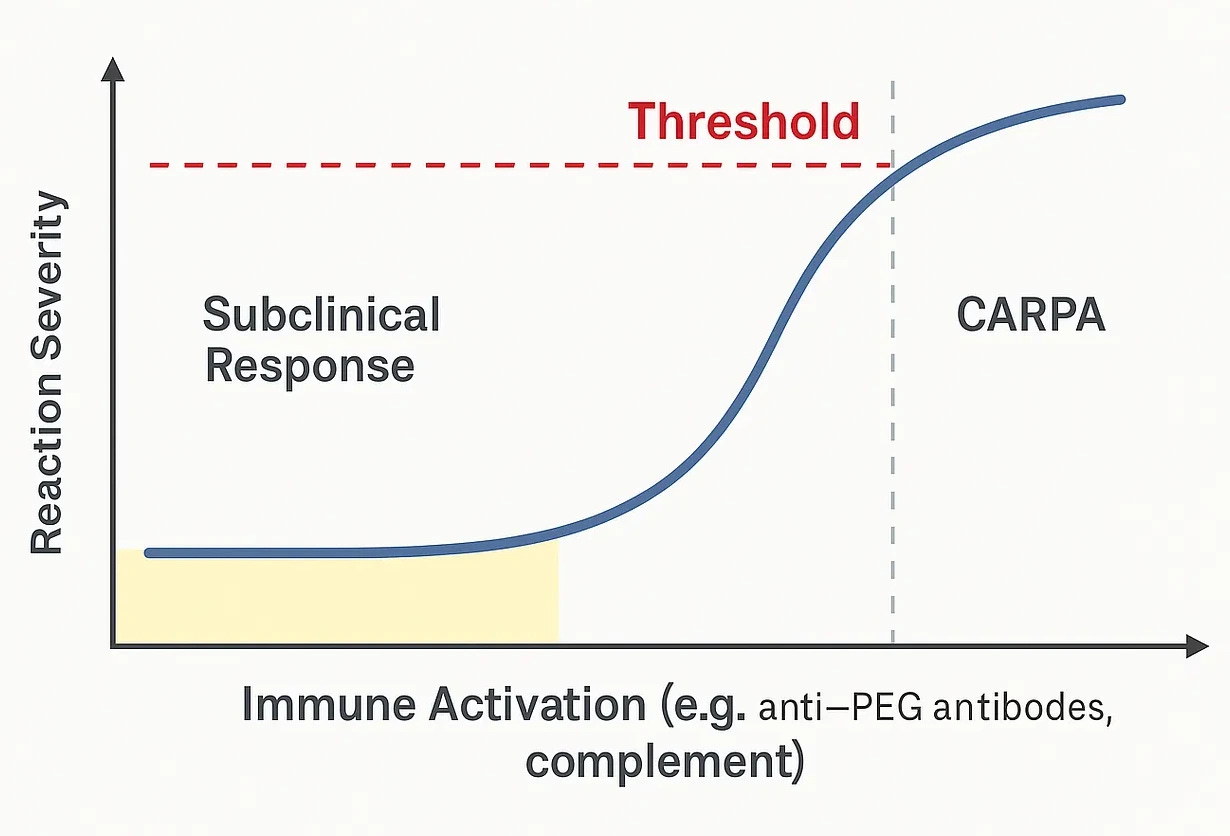
Threshold Response
CARPA is an all or nothing response. This occurs when complement activation surpasses a critical level. This makes CARPA fundamentally different from classical dose-dependent toxicity.
Subclinical
at low levels of anti-PEG antibody titers, complement activation can occur silently, without symptoms
these subclinical reactions may still contribute to mild inflammation or immune priming but go unnoticed
Critical Point or Threshold
once the ACTIVATION of the complement components (C3a, C5a and membrane attack complex) exceeds the threshold for the particular patient, then there is a systemic pseudoallergic response
this is influences by the antiPEG IgM/IgG titres, pre-existing inflammation, individual sensitivity, LNP dose and RATE OF ADMINISTRATION
PS This is why I think the issue with accidental IV administration of the mRNA vaccines causes serious AEs because the threshold for CARPA happens earlier. It is the RATE or BOLUS which can trigger CARPA.
Which is why we always slow down infusions if we see a CARPA or infusion related reaction in hospital
Rapid Escalation after the Threshold is Reached
symptoms appear within minutes
the reaction is often self-limited (but not always if the reaction is severe) and at this point looks like anaphylaxis
Non-linearity
increasing the dose does not increase severity
rate of administration may decrease severity
OK, enough about CARPA and some of the clinical issues and their presentation. What about ISRR?
What Is ISRR?
ISRR refers to a cluster of transient symptoms triggered not by the vaccine components, but by stress associated with the immunization process. This includes:
anxiety-induced vasovagal syncope, i.e fainting
hyperventilation,
dissociative reactions,
sympathetic overactivation.
You know, the types of reactions particularly common in adolescents (young men!) and those with needle phobia.
Symptoms may include:
Dizziness
Numbness or tingling
Chest pressure or tightness
Hyperventilation or throat discomfort
Tachycardia or hypertension
Here is a handy paper telling clinicians how to handle these responses to “sustaining trust” in vaccines.
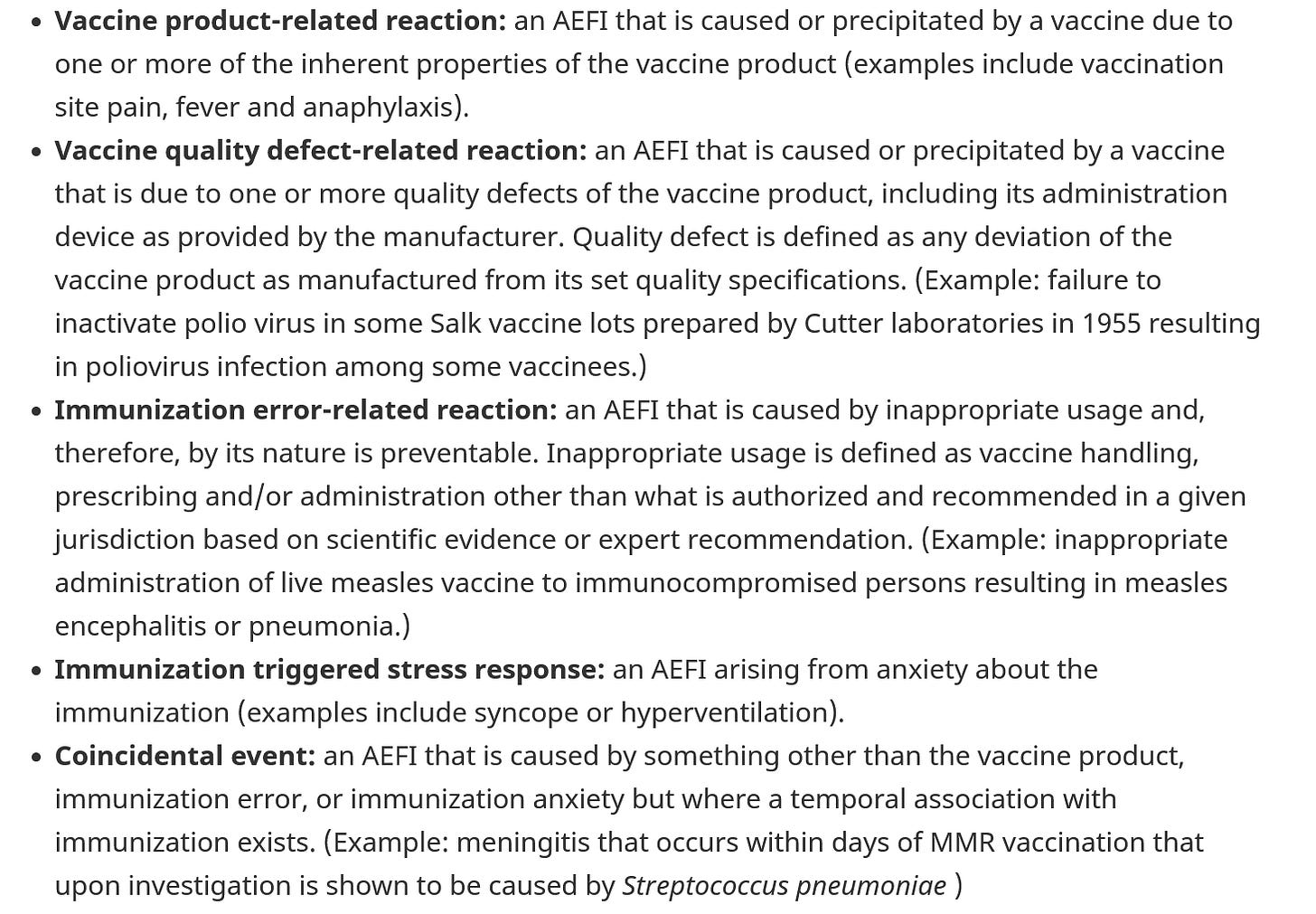
WHO Brighton Collaboration AEFI Definitions
You need to keep in mind, that AEFI as defined by the WHO and the Brighton Collaboration fall into 4 main buckets, and then the handy “coincidental event”. This is from Health Canada.
Here, we are looking at the Immunization Triggered Stress Response as mentioned above. It occurs due to ANXIETY about immunization.
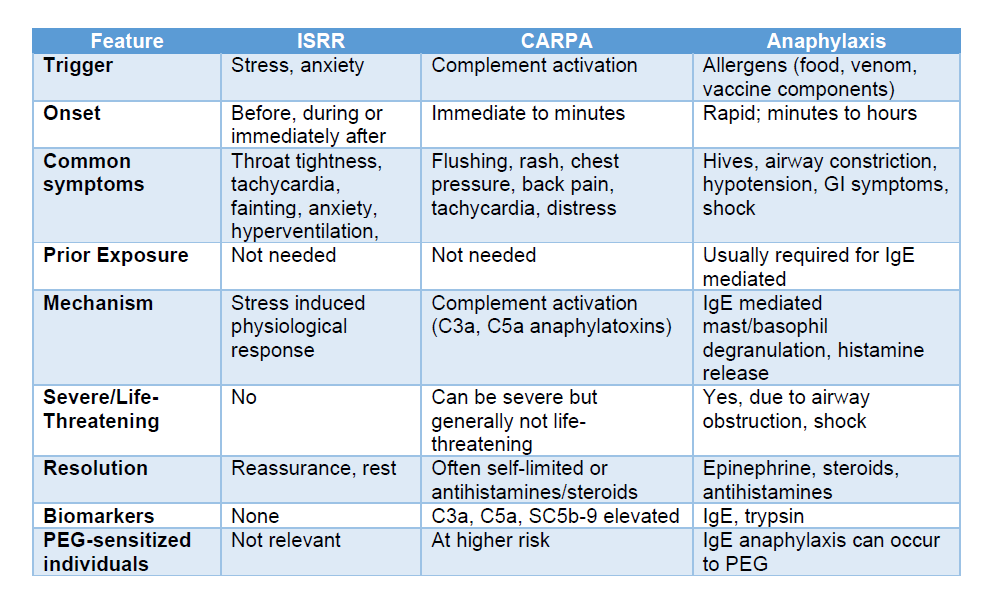
Symptom Overlap: Why the Confusion?
In the real world, these reactions can overlap because their symptoms are similar, both reactions can present within minutes of injection and resolve quickly. As a result mild CARPA is often indistinguishable from ISRR without lab testing.
Anaphylaxis is better delineated and known by clinicians, so less likely to be confused with CARPA, but it happens. I see this sometimes in hospital patients.
Here is a Table I did, that lays it all out
The Brighton Collaboration and Diagnostic Drift?
I believe this WHO’s Brighton Collaboration definition of IRSS acts as a non-immunologic diagnostic bucket for vaccine-related events. While perhaps useful for managing mass immunization campaigns (when there is not time to figure out what exactly is happening), the framework may encourage a reliance on psychological explanations for physiological symptoms especially since it appears not many clinicians know about CARPA and as a result, there are no complement or cytokine assays.
If Public Health is classifying many early-onset events as stress-related, then biological reactions with real mechanistic pathways like CARPA and the safety implications, are overlooked. And I believe that is a problem.
If mild CARPA is being misclassified as ISRR, several potential consequences follow:
Underreporting of true adverse events: Safety signals from innate immune activation may be missed. Ya think?
Patient mistrust: Individuals dismissed as "anxious" may feel gaslit or invalidated.
Regulatory blind spots: CARPA is not monitored in VAERS or EMA systems under its own code. In fact, the EMA asked for C3a and C5a assays but because no CARPA was noted in the Phase III trial, nothing more was done.
Formulation risks ignored: PEGylated and ionizable lipids may be inherently reactogenic. And that risks LNPs for the future, as I discussed in my previous substack.
Scientists from NIAID wrote a paper called
The conundrum of COVID-19 mRNA vaccine–induced anaphylaxis
NOT ONCE DO THEY MENTION CARPA IN THE ABSTRACT AND THEY SHOULD KNOW. All those allergic-type ADRs which are not clearly anaphylaxis are really just ‘psychogenic.’ This is what I mean by diagnostic drift.
In the figure below, they have complement activation but it is rolled up into “vaccine-induced anaphylaxis” and not in a separate category. This implies CARPA is similar to anaphylaxis and should be managed the same. Milder to moderate cases of CARPA would then likely be classified as ISRR, imho.
What Clinicians and Patients Should Know About Early Onset Reactions
Not all immediate reactions are psychogenic or just anxiety. Even if Public Health thinks so.
CARPA requires no prior sensitization and can occur on first exposure.
Diagnostic tools (e.g., complement assays, anti-PEG IgG/IgM panels) are available but rarely used.
Premedication strategies used in oncology (e.g., corticosteroids, antihistamines) may mitigate risk in known reactors.
Repeat dosing may increase risk if anti-PEG antibody levels are boosted by the first dose.
Recommendations
We need education on CARPA. Educate clinicians, especially pharmacists (who should have figured it out) and doctors on CARPA as a differential diagnosis.
Update adverse event protocols to include complement-related pathways. Include it in the VAERS reporting as a specific entry. Provide information on management and subsequent steps.
Encourage diagnostic testing in post-vaccination reactions, especially those with cardiopulmonary features. This will differentiate CARPA from ISRR.
Refine WHO classification to clearly distinguish ISRR from innate immune-mediated reactions. Why was ISRR added in the first place? How about getting rid of WHO classification for AEFI for vaccines?
Don’t gaslight patients. Support patients with good information, validation and diagnostic follow-up, not dismissal. Maybe some of the ISRR reactions due to “anxiety” in young men for example, might have been myocarditis?
Thank you for reading and as always, pray the rosary



Hooray! Congrats on the rebrand, I love it!
I now realise the naming of anything is important, it helps identify the aim, goal or objective of the project. You have shown yourself to be an independent thinker who worked within the system and is using all of your past collective knowledge to dive into the possible and actual effects of the mRNA technology on humanity. The Offscript Pharmacist is perfect for this still unfolding situation.