Health Canada, SV40 and ATIPs: Part 2 September to October 2023
Insights from the various ATIPs and an in-depth analysis
This is a continuation of Part 1 which documents the ATIPs at Health Canada and the presence of SV40 sequences. I’ve been asked to write a brief Executive Summary which I am generally reluctant to do, since you can lose detail and context, and then these statements can be used against the writer. However, we wrote a very short summary. Please scroll to the end.
Thank you again to Karen Rucas who organized the emails by data and pointed out some important changes in the drafting of Health Canada responses to media.
In Part 1, we left at the completion of the XBB vaccine review by the Quality division of the Centres Vaccines, Clinical Trials and Biostatistics (CVCTB)
1. Dr Phillip Buckhaults Presents to the US South Carolina Senate
September 12, 2023
This video was shared widely on social media. I suspect Health Canada was also aware of Dr Buckhaults’ presentation. Dr Buckhaults does a lot of sequencing of the human genome and what can lead to cancer and what does not. He calls himself a “cancer gene jockey.”
Video of Dr Buckhaults presentation
He explains how pieces of naked DNA allowed in protein vaccines at a certain threshold were not so problematic in a different era but with encapsulation in liposomes/nanoparticles, they can now easily get into cells. If they get into cells they can integrate in the genome which is permanent, long lasting, and has a theoretical risk of causing cancer depending on where in the genome they integrate. He says short pieces of DNA are analogous to buckshot and therefore can increase the hazard of genomic integration.
These are his short and well explained slides that he used at that presentation. Worth viewing, imho.
2. Health Canada Officially Approves the XBB 1.5 Vaccines
September 12, 2023
Dr Supriya Sharma gives a Press Conference on the approval of the Moderna XBB 1.5 vaccine ONLY. Regarding the Pfizer vaccine she says that Pfizer (and Novavax) are being reviewed on a priority basis and that there are “many factors” which could influence the timing of their approval. She also states HC uses the most robust evidence ONLY from “trusted and reliable” sources. Well, could it be that Kevin McKernan is a trusted and reliable source since his work was included in Pfizer’s XBB vaccine assessment?
September 28, 2023
Health Canada approves Pfizer’s XBB 1.5 vaccine.
I find the fact that there was NO Press Conference for the approval of the Pfizer XBB 1.5 vaccine very telling.
3. The EMA Reaches Out Pfizer and Then to Health Canada and the FDA Regarding DNA levels and the SV40 Sequences
October 12, 2023
What is your perspective? Well I think the EMA knows what HC’s perspective is so I believe this is directed to the FDA. And has anyone taken action? Does the EMA know what HC has done so far? Also, what about you FDA? And who are the EXTERNAL PARTIES? McKernan? Buckhaults? Scientists in Germany? Media?
October 13, 2023
The FDA responds, very early in the morning.
Tracking all these external parties discussion?? Probably the fallout from Buckhaults’ testimony is my guess. Things are heating up. Notice, “if helpful” line.
The EMA concurs, and would like the FDA on board.
Note the concern about “navigating the communication challenge.” It’s the COMMUNICATION CHALLENGES that is the real issue. Not safety?? Though I can appreciate that media and other inquiries can be a challenge when you are working in government. Maybe it’s a way to get the FDA involved?
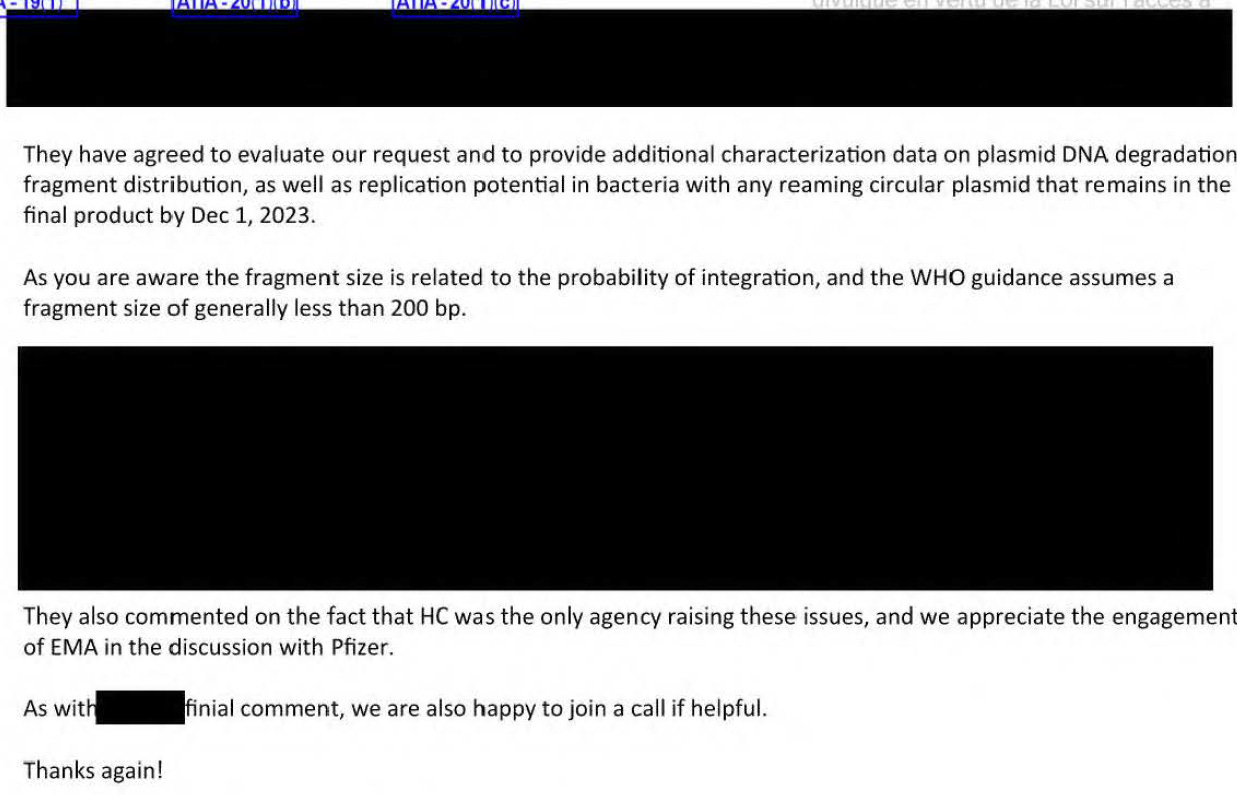
Dean Smith then updates his contacts in the FDA and EMA on what HC has been doing.
HELLO. They are talking about the probability of integration and those DNA fragment sizes. But what is in those redactions? The removal of the SV40 sequences? Anything else? Dean Smith informs the FDA of Pfizer’s gaslighting we saw previously in Part 1. “They commented on the fact that HC was the ONLY AGENCY raising these issues…” and that the involvement of EMA in discussions with Pfizer is appreciated, hinting that having FDA on board will be important. The FDA knew since June 2023, and still in October it appears they are dragging their feet. WHY?
(and they forgot a redaction. David is the EMA contact?….)
4. Pfizer Inc Sends a Letter to the South Carolina Senate
October 16, 2023
Maybe the reason the FDA is somewhat reluctant is this letter by Pfizer, which has to be read in full to be appreciated. Holy Toledo.
The Committee heard remarks “that are incorrect” that the vaccine contains plasmid DNA that could be a theoretical cancer risk. Well there are no intact plasmids in the vaccine that Kevin McKernan could find. This is true, but besides the point. It is the DNA FRAGMENTS. Then they trot out the WHO and FDA guidelines and how they are meeting those 10ng/dose guidelines. And NOTHING about the SV40 sequences.
Does anyone else finds this letter just a bit intimidating?
5. Meeting with the EMA and FDA on the SV40 Sequences
October 16, 2023
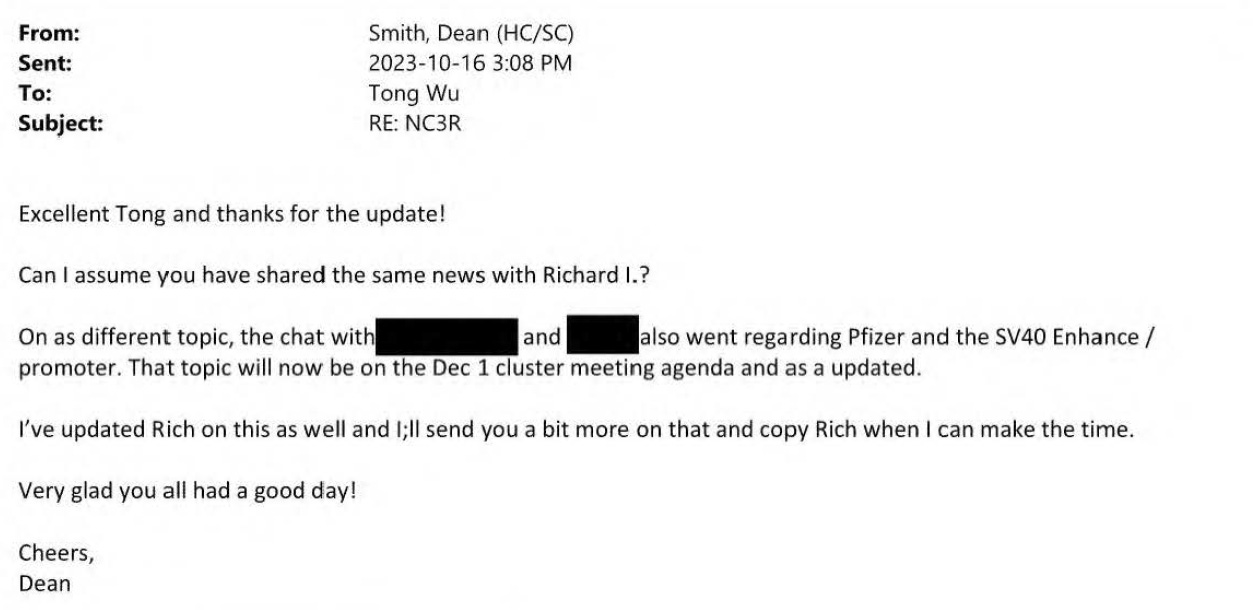
A meeting must have occurred shortly after those emails because Dean Smith reports back that it went well.
So finally all the major regulators are aligned and will be discussed at the next Cluster Meeting December 1st. Remember, that is also the date Pfizer was due with their report on the DNA fragment size, possible integration in bacteria and whether there were any intact plasmids etc etc.
Dean Smith tells his boss Co Pham that HC is further along discussing with Pfizer than either the EMA or FDA.
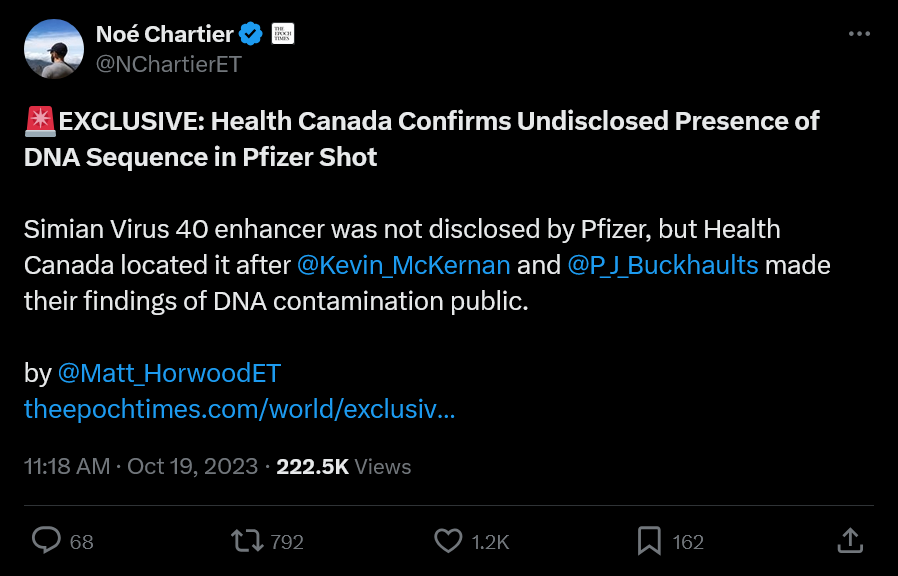
7. Noé Chartier and Matt Harwood Publish their Report on the DNA ‘Contamination’ and the SV40 Sequences in Epoch Times
October 19, 2023 at approx 11:00AM
EXCLUSIVE: Health Canada Confirms Undisclosed Presence of DNA Sequence in Pfizer Shot
Noé also drops the emails we saw in Part 1 to authenticate what he was reporting since many scientists were in disbelief.
8. Health Canada is Flooded with Inquiries from News Agencies Throughout the World
October 19, 2023
By 2pm on October 19th, 2023 Health Canada has received a large number of media requests on the Epoch Times article. I guess media does read Epoch Times.
Here is Reuters Fact-Checking. It can’t be true, can it Health Canada?
On the other hand, the British Medical Journal asks all the right questions.
Well I checked BMJ. Nothing regarding the contamination or residual DNA in the vaccines that I can find on BMJ Open website.
And Reuters Fact Check wrote an article which states Posts misrepresent Health Canada statement on DNA in Covid vaccines
The organization points to an October 19 article from the Epoch Times, a website that has previously spread misinformation about vaccination and the pandemic.
The claims stem from what experts say is a flawed paper following up on a similar report from earlier in 2023 -- neither of which is peer-reviewed.
"The preprint paper cited here has numerous methodological and interpretation issues," said Pakes of the University of Toronto. "I would certainly wait until it was published in a peer-reviewed journal before giving it any credence."
A little mendacious, wouldn’t you say? Co Pham, the head of CVCTB at Health Canada is much fairer.
9. Speicher et al Publishes Their Preprint on the Same Day
October 19, 2023 available HERE
10. Noé Chartier has a Few More Questions Regarding the Speicher Paper
October 23, 2023 Chartier sends his questions and the Speicher preprint to Health Canada. Here are the questions and the draft responses. You can also see how Co Pham and their group revised the answers to be accurate and detailed.
Note the issues regarding Qubit (ie a fluorometry method) and the fact that the measurements are more than 2,000 fold difference. They do not believe that fluorometry should be used from previous experience. Also note they no longer talk about the 1 TRILLION copies of the DNA template to make the modRNA which is obviously incorrect (its a lot more and then the template is broken up into more pieces).
Co Pham chastises the Health Canada drafters’ response and says, regarding the fact that it is not peer reviewed:
“we cannot be dismissive here” “we need to be precise in responding”
Forget about plasmids….it is the DNA FRAGMENTS WHICH ARE PROTECTED BY THE LNPs. “should we not have a line?” He gets it.
I like this guy.
This is the final response. “…Health Canada will not issue any public statement”.
10. Pfizer Provides its Response to the Request of Health Canada on the Size Distribution of the DNA Plasmid Fragments and Possible Integration of Plasmid in Bacteria
October 20, 2023
While trying to answer all the media requests, Pfizer submits the required report that was due December 1st, EARLY. Unfortunately most of this 56 page report is redacted. However, there are a few points that I would like to comment on.
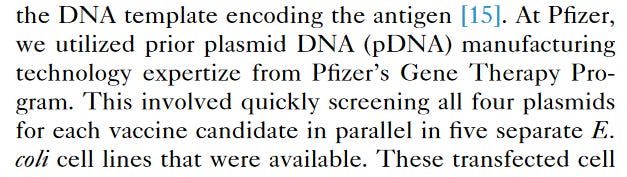
A MULTIPURPOSE PLASMID? Multipurpose plasmids containing SV40 sequences? Is it because one of its ‘purposes’ includes using this plasmid as gene therapy? Pfizer admits it themselves, in a paper published in 2022
PFIZER USED A MULTIPURPOSE PLASMID FROM ITS GENE THERAPY PROGRAM. HOLY TOLEDO. Is this a de facto admission that the vaccine is gene therapy?
It gets worse. They try justifying that the DNA in the LNPs do not get into the nucleus. I am pretty certain that Co Pham and his group asked Pfizer for this assessment regarding the possibility of the DNA fragments getting in the nucleus. This is part of Pfizer’s answer.
What is really ironic, is that reference 12, is written by David Dean in 2004 notes
This result suggests that SV40 DNA contains some element that allows preferential nuclear import of the DNA. Indeed, when as little as 50 bp of the SV40 enhancer was cloned into these other plasmids, they were able to enter nuclei with the same kinetics as the entire SV40 genome
PFIZER USED A REFERENCE THAT SHOWS THE SV40 ENHANCER ALLOWED NUCLEAR IMPORT EVEN IN NON-DIVIDING CELLS AS JUSTIFICATION that DNA fragments in the cytoplasm, are not a risk.
I have no words.
For a further more in depth discussion, please see:
Note: A complaint has been filed with Office of the Information Commissioner (IOC) to get this ATIP unredacted. Stay tuned.
11. The US CDC Gets Involved, Tries to be ‘Helpful’ with ‘Messaging.’
October 26, 2023
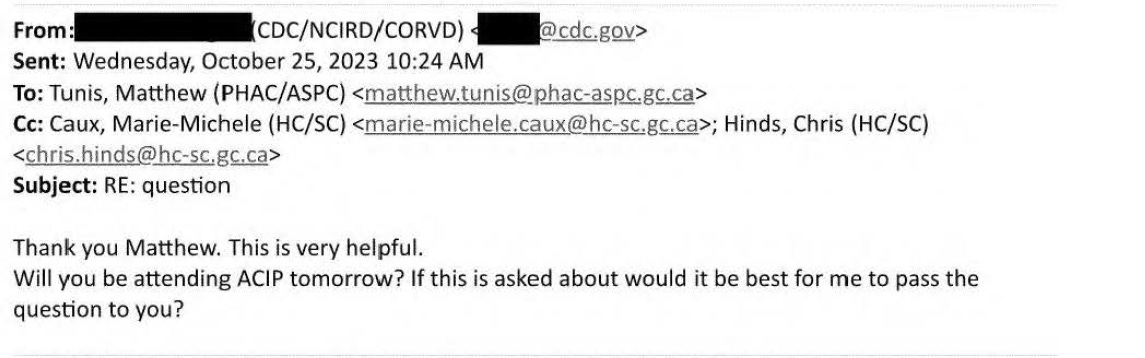
Well the reporting and admission of the presence of the SV40 sequence has reached the Centres of Disease Control (CDC), and they contact Matthew Tunis at the Public Health Agency of Canada (PHAC), the Canadian equivalent of the US CDC regarding this RioTimes article
Matthew Tunis is the Executive Secretary of the National Advisory Committee on Immunization (NACI) which is the committee which makes and advises on immunization practices in Canada. The American equivalent would be the Advisory Committee on Immunization Practice (ACIP). Note the “if it exists” comment.
Matthew answers his CDC contact (which turns out to be the LEAD at ACIP…)
Oh, it’s accurate but lacking ‘context’. As if the adenovirus virus sequences have anything to do with the mRNA vaccines. And that no ‘peer reviewed literature’ regarding fragmented DNA which Pfizer itself admitted is possible, but at very low rates. Co Pham at the CVCTB was much more honest and scientific. Matthew tells his contact, that the Communications branch is preparing a response to all the media outlets which the CDC might like.
The CDC contact is worried about the ACIP meeting coming up and want to direct any questions to Matthew so that the context can be maintained.
Matthew Tunis plans to give LINES TO THE CDC so they ‘can get the story straight’ and provide context (so they can downplay the admission by Pfizer, etc)
Now the CDC then tries to give LINES TO Matthew Tunis at PHAC. OMG. Really.
Safe and Effective. Safe and Effective. Keep it broad. Doesn’t matter about the SV40 sequences, the amount of DNA fragments, the fact Pfizer lied. Nope. Safe and effective and you Canadians should make sure to stay in line.
But SORRY, NOT SORRY, Marie-Michele Caux, the Director of Public Health Communications at PHAC tells the CDC and Matthew Tunis that the communique won’t be ready (so there won’t be any ‘lines’ to give the CDC).
Was Health Canada concerned that perhaps the CDC would hijack their responses?
12. Health Canada Crafts the Response to Media/CDC Inquiries
This actually took some time. First of all, the rest of Health Canada, specifically PHAC and Dr Supriya Sharma, Chief Medical Advisor to Health Canada, needs to understand the basic technical aspects of these findings.
October 26, 2023 09:53
So this is what Co Pham, the Director of the CVCTB, or Vaccine Division is telling his superiors.
SV40 promoter/enhancer DRIVES GENE EXPRESSION
there is no real purpose for it to be there to manufacture the COVID-19 vaccine (he likely knows the AmpR promoter is already there?)
This promoter HAS NEVER BEEN USED IN THE MANUFACTURING OF VACCINES
You can’t remove all the DNA fragments but you CAN get rid of the SV40 sequences
Oh, and what is that redacted portion? That Moderna didn’t need the SV40? The presence of the AmpR promoter and other sequences ? Any other ideas?
Keep these points in mind when you see the final communique or response.
October 26, 2023 1:50 PM
Marianne van Oosten, who is from Communications and Public Affairs at Health Canada (and NOT AT PHAC) drafts a statement on the SV40 DNA fragments.
Health Canada authorized the use of the first Pfizer-BioNTech COVID-19 mRNA vaccine in December 2020 and has authorized several versions of the updated vaccine since then. Each time, Health Canada determined that the vaccine meets the Department's stringent safety, efficacy and quality requirements for use in Canada, and we continue to have full confidence in these vaccines. (THIS WILL BE EDITED OUT IN SUBSEQUENT DRAFTS)
As a regulator of vaccines, Health Canada sets quality standards and requirements for manufacturers to follow, including providing comprehensive and detailed information about the vaccine itself, and about the manufacturing process. In the manufacture of any vaccine, it is expected that there may be variability or residual elements that are produced during the manufacturing process. To manage this, Health Canada has strict limits and controls for the presence of these residual fragments to ensure that the vaccine continues to be safe, and that any residual fragments are both inactive and have no functional role in the vaccine.
In the case of the Pfizer-BioNTech Covid-19 vaccine, the SV40 promoter enhancer was found to be a residual DNA fragment of the manufacturing process, however it is inactive, has no functional role, and was measured to be consistently below the limit approved by Health Canada, which is not more than 10 ng/human dose. This is in line with the World Health Organization's recommendation concerning residual DNA in biological drugs.
Health Canada confirms that the presence of the SV40 promoter enhancer as a residual DNA product does not affect the safety or efficacy of the Pfizer-BioNTech Covid-19 mRNA vaccines and does not change Health Canada's benefit-risk assessment for the vaccine's use.
With respect to unsubstantiated reports of the simian virus 40 in vaccines and a potential link to cancer, we wish to emphasize that there is no evidence to indicate that these Covid-19 vaccines cause or accelerate cancer. To be clear, the vaccine does not contain the SV40 virus; the presence of the SV40 promoter enhancer sequence is not the same as the presence of the whole virus. Monitoring of the residual DNA fragments is done by the manufacturers using methods that have been reviewed and validated by Health Canada as appropriate for its purposes. All Pfizer-BioNTech's Covid-19 vaccine commercial batches released in Canada complied with the requirements approved by Health Canada, including the residual DNA.
It is precise and fairly clear. The context of “inactive” and no “functional role” is with respect to not being required in the plasmid as we see Co Pham explain earlier, but it gets watered down here and it appears HC is saying the SV40 sequences have no activity at all. The presence of the sequences does not affect the benefit risk calculations???? Not sure the vaccine scientists see it this way.
October 26, 2023 2:06 PM
Well, yes indeed, Co Pham suggests further edits.
Health Canada authorized the use of the first Pfizer-BioNTech COVID-19 mRNA vaccine in December 2020 and has authorized several versions of the updated vaccine since then. Each time, Health Canada determined that the vaccine meets the Department's stringent regulatory safety, efficacy and quality requirements for use in Canada, and we continue to have full confidence that the benefits outweigh the risks for these vaccines. As a regulator of vaccines, Health Canada sets quality standards and requirements for manufacturers to follow, including providing Health Canada authorized the use of the first Pfizer-BioNTech COVID-19 mRNA vaccine in December 2020 and has authorized several versions of the updated vaccine since then. Each time, Health Canada determined that the vaccine meets the Department's stringent regulatory safety, efficacy and quality requirements for use in Canada, and we continue to have full confidence that the benefits outweigh the risks for these vaccines. As a regulator of vaccines, Health Canada sets quality standards and requirements for manufacturers to follow, including providing comprehensive and detailed information about the vaccine itself, and about the manufacturing process. In the manufacture of any vaccine, it is expected that there may be variabilities or residual elements that are part of the standard manufacturing process. To manage this, Health Canada requires strict quality limits and controls for the presence of these residual fragments to ensure that the vaccine continues to be safe, and that any residual fragments are both inactive and have no functional role in the vaccine.
In the case of the Pfizer-BioNTech Covid-19 vaccine, the SV40 promoter enhancer was found to be a residual DNA fragment in the vaccine, however it is inactive, has no functional role, and was measured to be consistently below the limit required by Health Canada for approval, which is not more than 10 ng/human dose. This is in line with the World Health Organization's recommendation concerning residual DNA in biological drugs, and consistent with the quality limits by other international regulators. Pfizer has confirmed with Health Canada that the presence of the SV40 promoter enhancer as a residual DNA product that does not affect the safety or efficacy of the Pfizer-BioNTech Covid-19 mRNA vaccines and does not change Health Canada's benefit-risk assessment for the vaccine's use.
With respect to unsubstantiated reports of the simian virus 40 in vaccines and a potential link to cancer, we wish to emphasize that there is no evidence to indicate that these Covid-19 vaccines cause or accelerate cancer. To be clear, the vaccine does not contain the SV40 virus; the presence of the SV40 promoter enhancer sequence is not the same as the presence of the whole virus. Monitoring of the residual DNA fragments is conducted by the manufacturers using methods that have been reviewed and validated by Health Canada as appropriate for its purposes. All PfizerBioNTech's Covid-19 vaccine commercial batches released in Canada complied with the requirements approved by Health Canada, including the residual DNA.
Happy to discuss.
Thanks
C
Co Pham makes sure the “full confidence” is moderated by the “benefits outweigh the risks” line. Also, see how he says Health Canada “requires…that any residual fragments are both inactive and have no functional role in the vaccine?” Not that Health Canada has determined that to be true? And he says that it is PFIZER, who says the SV40 promoter/enhancer does not affect the “safety or efficacy” of the vaccine? He is being very very careful here. No evidence these particular vaccines cause or accelerate cancer? Yes, strictly true, no current evidence, but no discussion either that there is a potential RISK for cancer.
October 26, 2023 3:09
Well that response does not fly with the Communications Branch. Here is the final draft sent out to all the 12 media outlets asking about the Epoch Times article on the 19th of October, 2023
Health Canada initially authorized the Pfizer-BioNTech COVID-19 mRNA vaccine in December 2020 and subsequently has authorized updated versions, including the most recent vaccine targeting the XBB Omicron subvariant in September 2023. Each assessment included a determination that the vaccine met the Department's stringent regulatory safety, efficacy and quality requirements for use in Canada. As a regulator, Health Canada sets quality standards and requirements for manufacturers to follow, including providing comprehensive and detailed information about the vaccine itself, and about the manufacturing process. In the manufacture of any vaccine, residual elements that are part of the standard manufacturing process may remain. There are strict limits and controls for the presence of these residual fragments to ensure that there is no effect on the safety or effectiveness of the vaccine. The Pfizer-BioNTech COVID-19 vaccine does not contain simian virus 40 (SV40). The presence of the SV40 promoter enhancer sequence is not the same as the presence of the whole virus itself.
The SV40 promoter enhancer sequence was found to be a residual DNA fragment in Pfizer-BioNTech COVID-19 vaccine. The fragment is inactive, has no functional role, and was measured to be consistently below the limit required by Health Canada and other international regulators. Any claims that the presence of the SV40 promoter enhancer sequence is linked to an increased risk of cancer are unfounded. There is also no evidence to support that the presence of the full SV40 in any vaccine increases the risk of cancer or the acceleration of cancer in individuals.
Health Canada continues to monitor the COVID-19 vaccines to ensure that they continue to meet the highest standards for safety, effectiveness and quality and that their benefits continue to outweigh any potential risks.
I do not see Co Pham on the email header. Did he know of this Response released from the Communications Branch? Look at the lack of precision and the change in tone of this response. The SV40 sequence “was found”…note the passive tense. Not that Pfizer initially hid its presence and, when caught, later admitted to it. The inactive and no functional role is taken out of context with respect to manufacturing and is referred to as a DNA fragment that isn’t doing anything in the vaccine. And they also state that the “full SV40” in any vaccine does not increase or accelerate cancer. I am not so sure Co Pham and his team would agree with this statement without qualifiers. Holy Toledo. Here’s a comprehensive review of Simian Virus 40 and let’s not forget about Drayman et al who showed than the SV40 promoter binds to p53 considered the guardian of the genome.
Well at least there is no “we continue to have full confidence” in the vaccine.
13. Health Canada Sends Out Statement to the CDC
October 27, 2023 5:03 PM
The DAY AFTER the ACIP meeting, and late in the day to boot, Marie-Michele Caux, who is at Communications at PHAC, sends out the Response from Health Canada to the CDC AND Matthew Tunis. This was just after they had sent it out to all the media outlets who had asked for comment earlier. Yowza. What does everyone think? Do you read these emails the same way?
So does everyone think that there may be a bit of a struggle between Health Canada, especially the vaccine branch, and PHAC and/or CDC?
EXECUTIVE SUMMARY
Scientists at the vaccine division of Health Canada verify the presence of the SV40 promoter/enhancer immediately after finding out about it from Noé Chartier at the Epoch Times who sent them Kevin McKernan’s preprint from April 2023. They then initiate an Issue Analysis Summary (IAS) to investigate with the goal of removing these sequences and possibly restricting or removing the vaccine altogether. Several questions are sent to Pfizer for explanation and justification, but Pfizer tells Health Canada, they feel there is no problem and that no other regulator is asking these questions. Health Canada then takes the next 3 months trying to get the EMA and FDA on board. The EMA is willing but the FDA drag their feet until October (sometime after Dr. Phillip Buckhaults’ presentation to the South Carolina Senate, which went viral). Finally they agree that Pfizer needs to “remedy” the situation.
Pfizer submits the required documentation on the DNA fragments and role of the SV40 enhancer/promoter to Health Canada and admit they used a multipurpose plasmid (which could also conceivable be used for gene therapy). They also do not appear to present data regarding the risk of the DNA fragments and possible entry into the nucleus. In fact some of the references used as justification that there is no problem, actually show a risk. When Noé Chartier and Matt Harwood publish their report in Epoch Times, Health Canada is inundated with requests from media outlets to confirm this finding. On the same day, the second preprint by Speicher et al confirms the SV40 sequences and residual DNA in Ontario vials.
The CDC and PHAC try to provide “lines” to Health Canada which would mitigate and downplay these findings but Health Canada does not provide the CDC with the statement in time prior to the ACIP meeting. Emails show the amount of editing and crafting of the response by Health Canada Communications sent out to media and the CDC that altered the original intent and tone, though still confirming the presence of the SV40 sequences.
As for the outcome of the Issue Analysis Summary (IAS), that information is redacted and we know it was quashed. We are digging into that as well as having all of Pfizer’s report on the risk of DNA fragments and the SV40 sequences unredacted as well as an ATIP on that December 1st cluster meeting with the FDA and EMA. Wish us luck!
Thanks for reading, please share and oh Pray the Rosary.






































If this was not so frustrating and super important, I would just say "WOW." Given the implications, I want to scream. During the last few years I have seen several papers where the researchers used SV40 components to help drive stuff into the nucleus. It seems a commonly known step - other than when it involves the eugenic gene-therapy injections?!
There's utterly no evidence here that they are at all concerned with safety. The whole issue of safety is presumed. I guess they just delegated it to the company making billions off of the transaction. Nothing to see here.
Also, even those of us who are not biologists can figure out that regulations for traditional vaccines should not apply to these new biologics. They are entirely different types of products. Hence, saying "these drugs meet existing standards" is completely irrelevant.