The analysis of residual DNA in a High School Science Journal
A few observations
MaryAnne Demasi reports on an extra-ordinary paper.
This study was performed in a BSL-1 lab at the FDA White Oak Campus, by high school students under supervision of FDA scientists. This was published December 28th, and however Maryanne found it, or was tipped off to it, we thank her and her possible accomplices. Others have written on the pros an cons of this paper much better than I can. I just want to make a few observations that are bothering me for discussion.
Read Kevin’s great review. Congratulations to these high school students.
OK, here are some interesting observations of my own.
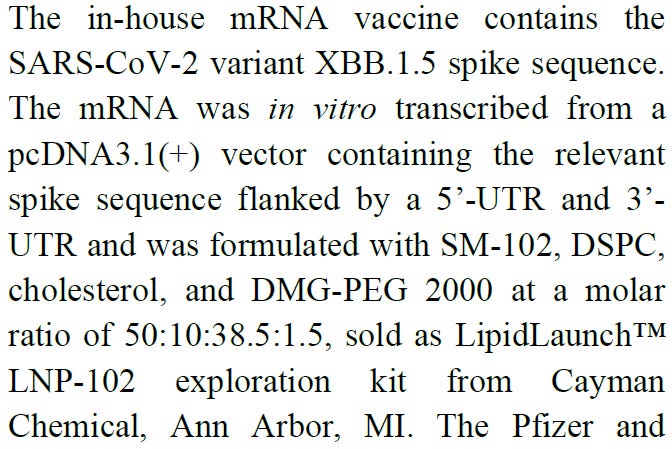
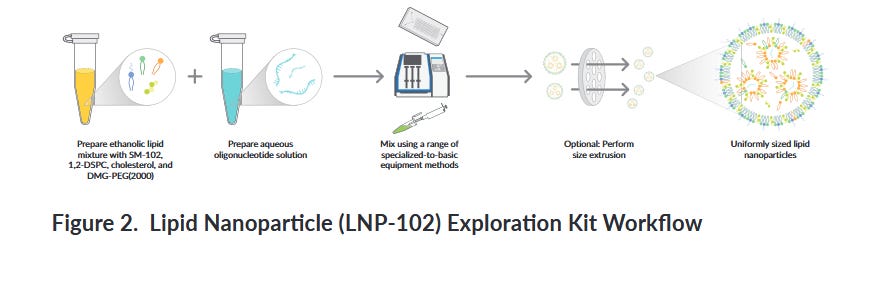
1. They made their own mRNA vaccine
So a common plasmid (vector) was used containing the XBB.1.5 sequence. The pcDNA3.1(+) vector is a mammalian expression vector with the CMV promoter (made by ThermoFischer or Invitrogen?) The MCS is in the forward (+) orientation. I don’t know what that means. Anyone?
It also has an SV40 promoter and SV40 ori, and an AmpR promoter and gene (instead of kanamycin). I THINK this is the right plasmid vector. If I have it wrong, please correct me in the comments. Did they grow this in E coli cells? I’m very confused.
Then they made their own vaccine using a kit. I am wondering where the IVT step went. This is the “kit” for Cayman Chemical
And here is the description. So you don’t need a T-mixer or a herringbone mixer or any specialized equipment. Who knew it was so easy to make mRNA/LNP products?
So this is using the Moderna ionizable and pegylated lipid in this kit. You can even hand mix them!! After that you better filter out the big LNP particles though because you won’t get a homogenous mixture.
Don’t try this at home, folks. They helpfully add, that it is not for human use. Well, neither was SM-102 when it was approved.
Still, where is the IVT step to make the oligonucteotide, or mRNA. They don’t mention it.
2. They got a hold of some COVID-19 “biosimilar” mRNA vaccines (WTH!!)
OK, to pharmacists and the industry, “biosimilar” means an mRNA covid vaccine NOT MADE BY PFIZER or MODERNA. Here is a quick and dirty explanation. For these vaccines, given the different manufacturing process, Process 2 Pfizer is a biosimilar to Process 1, ie another drug.
So what is this ‘biosimilar’ vaccine? Does the NIH have some frozen Process 1 vials lying around? Are these the early Process 2 vials approved under EUA (ie before Aug 2021 for Pfizer and Jan 2022 for Moderna)? Or are these trial vaccines made by another manufacturer or university?
BEI resources is an interesting website. You can order all kinds of reagents, pool blood with antibodies, anything you need to do infectious disease resources. Including mRNA vaccines. Weird. I couldn’t find the specific catalogue number on this site, but maybe I needed to register and be vetted. If anyone has used reagents etc from BEI resources, can they look to see if NR-59604 and NR-59605. Well none to be had, but they add helpfully, that MADE TO ORDER products are excluded. So maybe they were made to order? After all, there are kits to make your OWN mRNA products. Who knew?
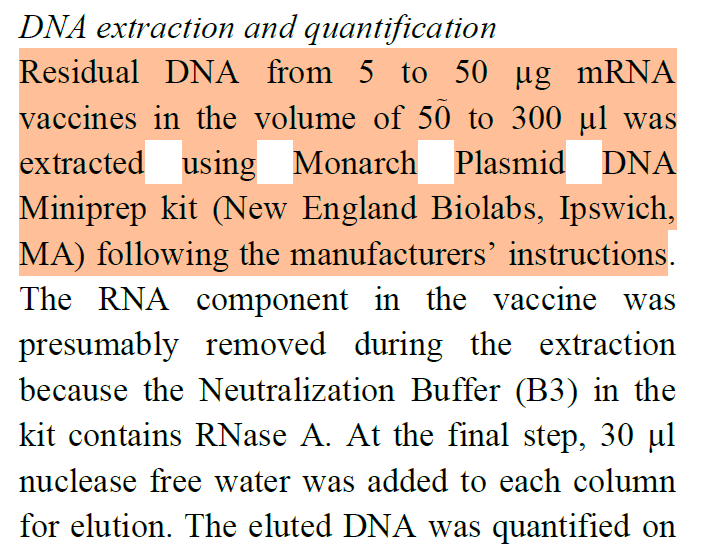
3. Extraction of the DNA from the mRNA vaccine
They use a DNA extraction kit to extract the residual DNA in the vaccines.
Kevin talks about what this kit does in his substack article for some of the problems he identified with this prep kit. Though is DOES have RNase A as part of the kit to get rid of any interfering RNA.
One problem I have however is,
WHAT DID THEY DO TO CRACK OPEN THE LNPS?
Did they do this step? Or are they just measuring the DNA outside of the LNPs in the sucrose/Tris formulation? I mean, this has been one of the hardest nuts to crack. Use Triton-X (a surfactant) like Kammerer used, or a boil prep like McKernan and Speicher used. Did they do anything?
4. How many total mRNA vaccines did they test?
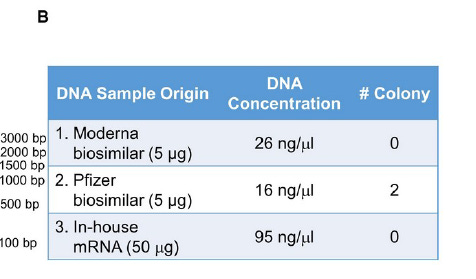
OK, so for determining replication competent DNA the used the in-house mRNA vaccine they made, plus the 2 “biosimilars” so 3 altogether.
Then after establishing this method they tested it in 2 commercial Pfizer lots.
Here they only report the PAA number but their picture shows the lot numbers.
If you do a search for PAA184098 you find this image (as did Geoff Pain)
This is the monovalent Pfizer vaccine with the trade name Comirnaty. The one above this is an older Pfizer vaccine WITHOUT the trade name…… could this be what they call a “biosimilar?”
If you do a search for the top vial, PAA194854 you get this, found on a Canadian site but I cannot find it in the Canadian National Vaccine Catalogue. It is the BA/4/BA.5 bivalent. A lot of people get the PAA and the Lot numbers mixed up in real life. It is not as obvious as you would think.
It looks like the students got the bivalent and movalent mixed up. It is VERY EASY to do so, and this has led to many administration errors in VAERS.
So a total of 5 vaccines. The picture of panel A of the thawing vaccines are just pictures of these 2 Pfizer lots. Wonder where they got them from? Most pharmacies would have gotten rid of these long ago so I think these were obtained from the federal stockpile. In Canada, all vaccines go to a central sorting station are logged then sent out to the provinces depending of what the province orders.
5. The FDA scientists who supervised this research won an award on a new COVID-19 vaccine
The scientists who supervised this research should be commended. They are
S Liu
P Selvaraj
T. Wang (possibly the father on Tyler Wang, one of the students?)
They won the 2023 US FDA Laboratory award for:
Here is the paper
So what are these FDA researchers doing developing a LAV??? Which wins an award? Does the FDA think that mRNA vaccines are a failure? What is going on?
6. has a very good take, imho
SUMMARY
This is a strange paper, imho. I agree with Siguna. THe conclusions can go either way, since they did not find replication competent DNA in the 2 Pfizer vaccine lots they studies, despite finding high total DNA levels via fluoroscopy.
I would like to know how they did the IVT for their home-made mRNA
And how they cracked open the LNPs, if that was done at all.
I am not sure this paper can be used to “get” the FDA, though the FDA supervisors are working on a TOTALLY different kind of vaccine.
Thoughts anyone?
Thanks for reading, and pray the rosary. This year will be crazy


















By "biosimilar" they mean the lots of the original EUA vaxxes, that are now de-authorized. The Pfizer lots shown are older lots, exp 2022. I will write a more in depth analysis on this. It seems like the FDA is scrambling to get ahead of this issue as they are afraid the investigation will begin under the new admin.
It's interesting in VAERS lot GL2042 is listed as BOTH mono- and bi-valent