As part of the the Ontario vials that we tested for DNA contamination, I decided to ask Health Canada for the lot release results for these particular vials.
1. My Request
This was my request:
Lot release testing for the drug substance is performed by the manufacturer and that is done upstream. So for residual DNA or dsRNA or percent purity of the RNA is usually not available and is communicated to the regulators through specific secure protocols.
However, lot release testing for the drug product can be done by external labs (like in the EU which uses OCML [Official Medicines Control Laboratories], the drug manufacturer, or the regulator or all three). It was a long shot since this is considered proprietary information and they don’t have to give me anything.
2. HC Informs Me One of the Vial Lots Does Not Exist
After I filed my request, the person handling my file emails me with this:
This was a Moderna lot. So I sent him this with a smile.
I really don’t blame the ATIP people or those trying to get the information. Some lots are very difficult to identify, and I have about 3 databases I use to find manufacturing and expiry dates. However, I think this little episode helped me a lot with my ATIP request since it was completed more or less within the time frame they gave me.
A first.
3. Pfizer Lots
As expected I did not get any ACTUAL values but they did give me the critical quality attributes that they tested and the analytic methodology.
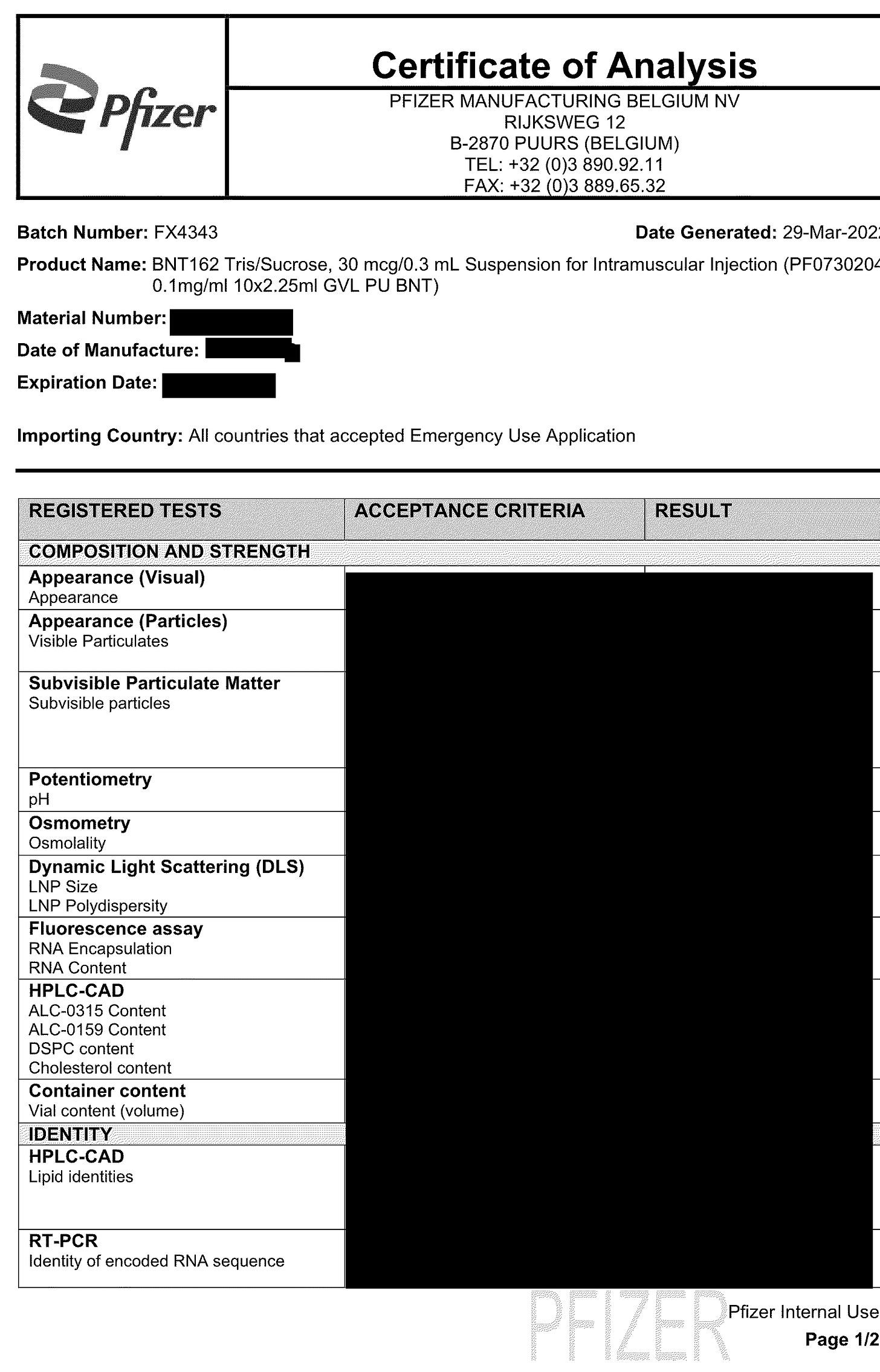
Here is Lot FX4343. This lot is a grey-topped monovalent vaccine, which came into Canada April 4, 2022 as per Canada’s National Vaccine Catalogue . This matches the date the Certificate of Analysis was generated.
A couple of things to note
This is the Tris formulation or as I like to call it Process 3 which you can read more about HERE.
See the Fluorescence Assay? It is being used to determine encapsulation rate AND the RNA content in the final drug product. This is what Kevin McKernan talks about. Fluorescence for RNA content which overestimates RNA but qPCR for the DNA content which underestimates total DNA.
And to determine if the mRNA construct in the final drug product, is the right mRNA, they use RT-PCR.
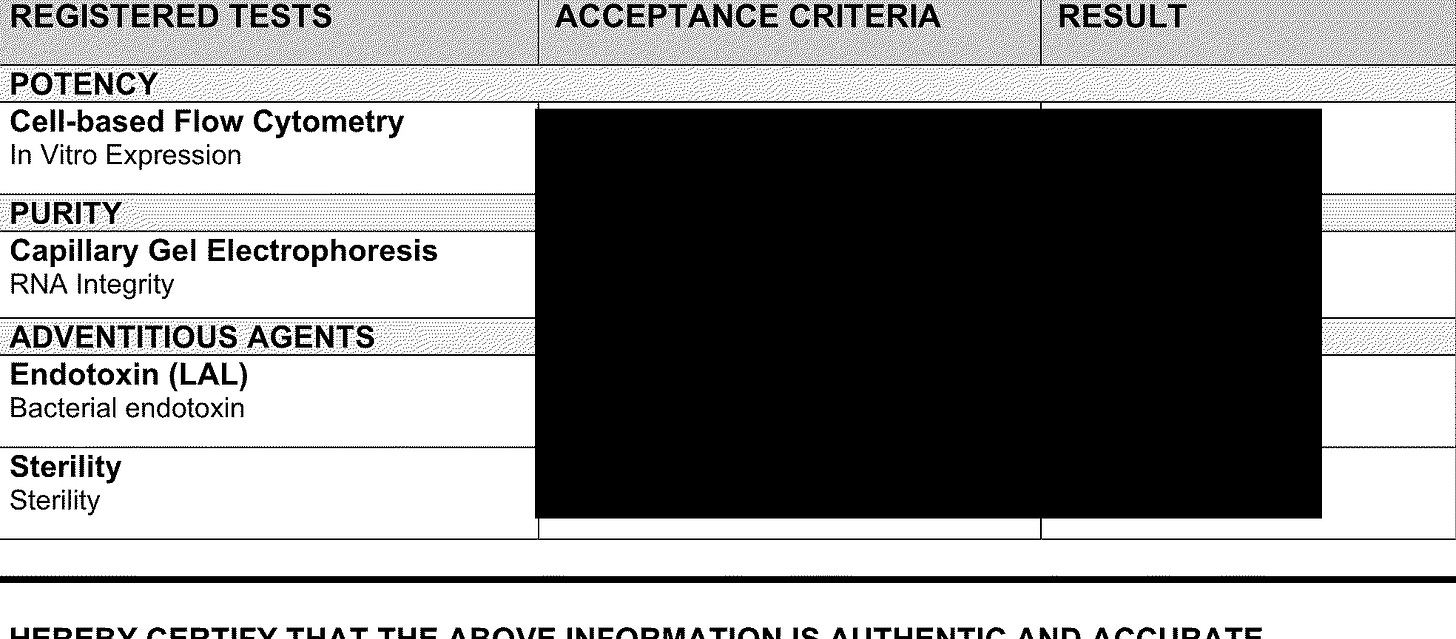
Ok, now for the XBB 1.5 lot
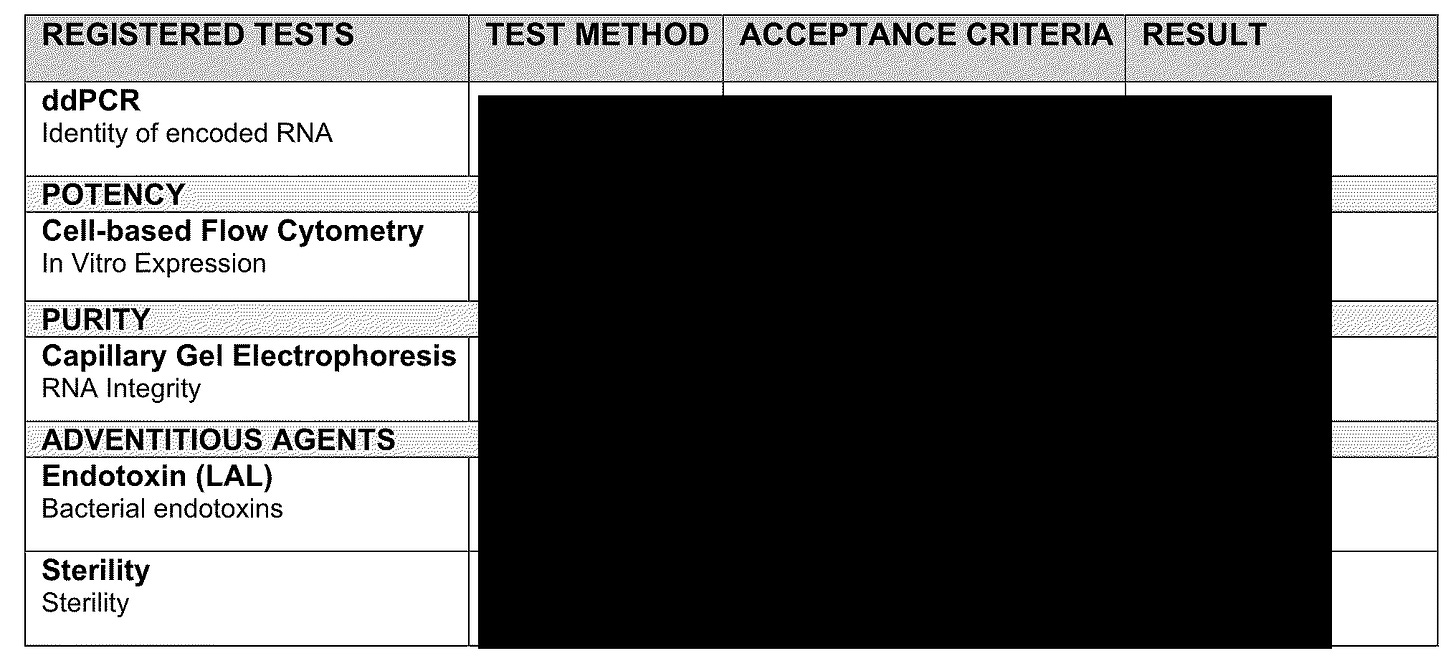
OK, the CQA’s or what they are measuring is the same but do you see “identify of encoded RNA”? It is now being measured by ddPCR instead of regular RT-PCR. HOWEVER, they are still using fluorescence to measure the quantity of RNA in the final drug product.
So why is Pfizer changing to ddPCR for measuring RNA identity? As per Grok, I think it is primarily because ddPCR doesn’t have issues with, say the LNPs. So why not use it for RNA quantification as well????? Can anyone tell me?
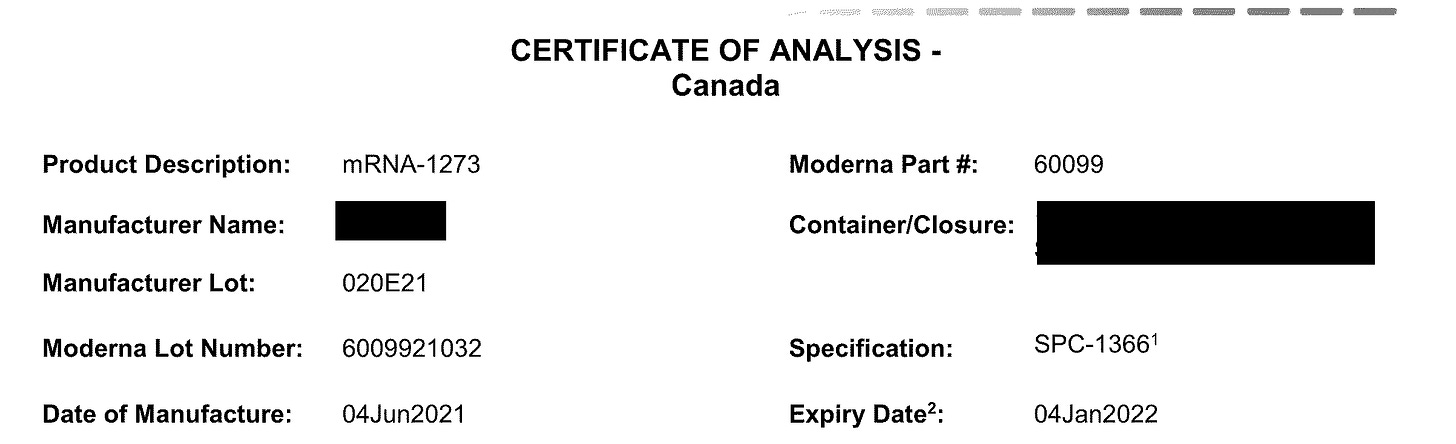
4. Moderna Lots
Here is when the fun starts. The earliest Lot we have is 020E21A.
Here is the Certificate of Analysis for this Lot.
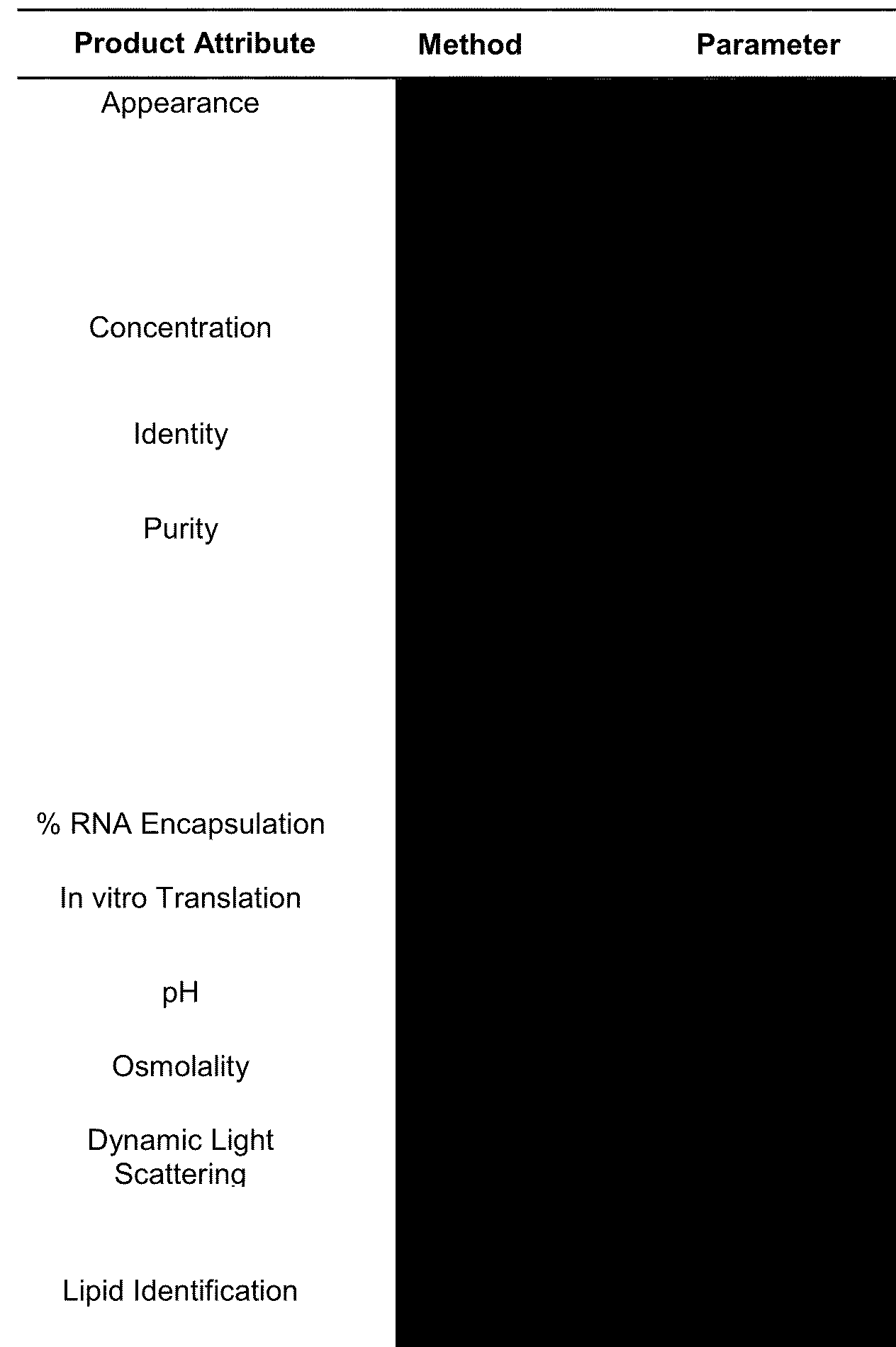
EVERYTHING is redacted but I do get what parameters they were testing for
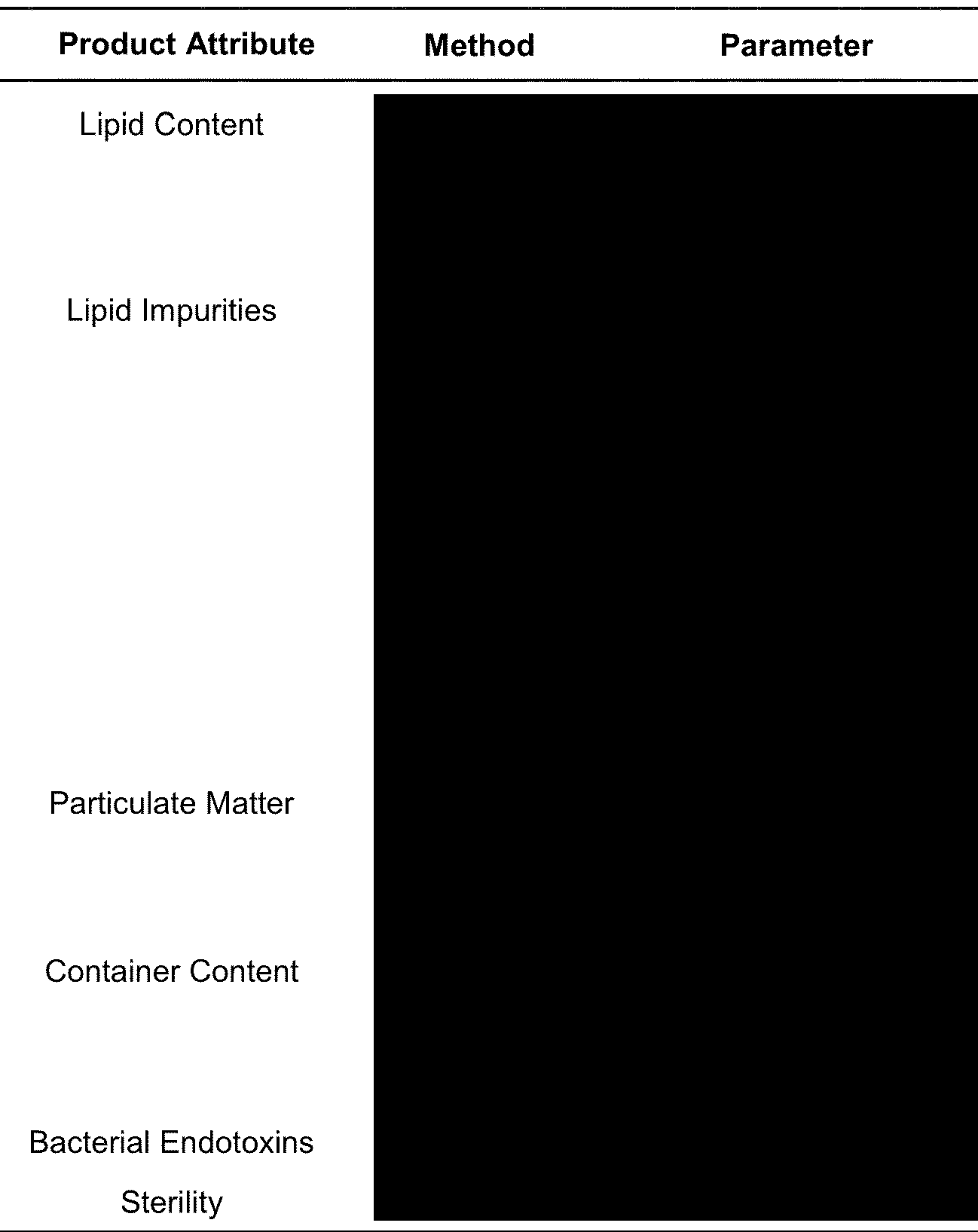
OK it looks the same as Pfizer except for this HUGE blackout area for LIPID IMPURITIES. What lipid impurities?
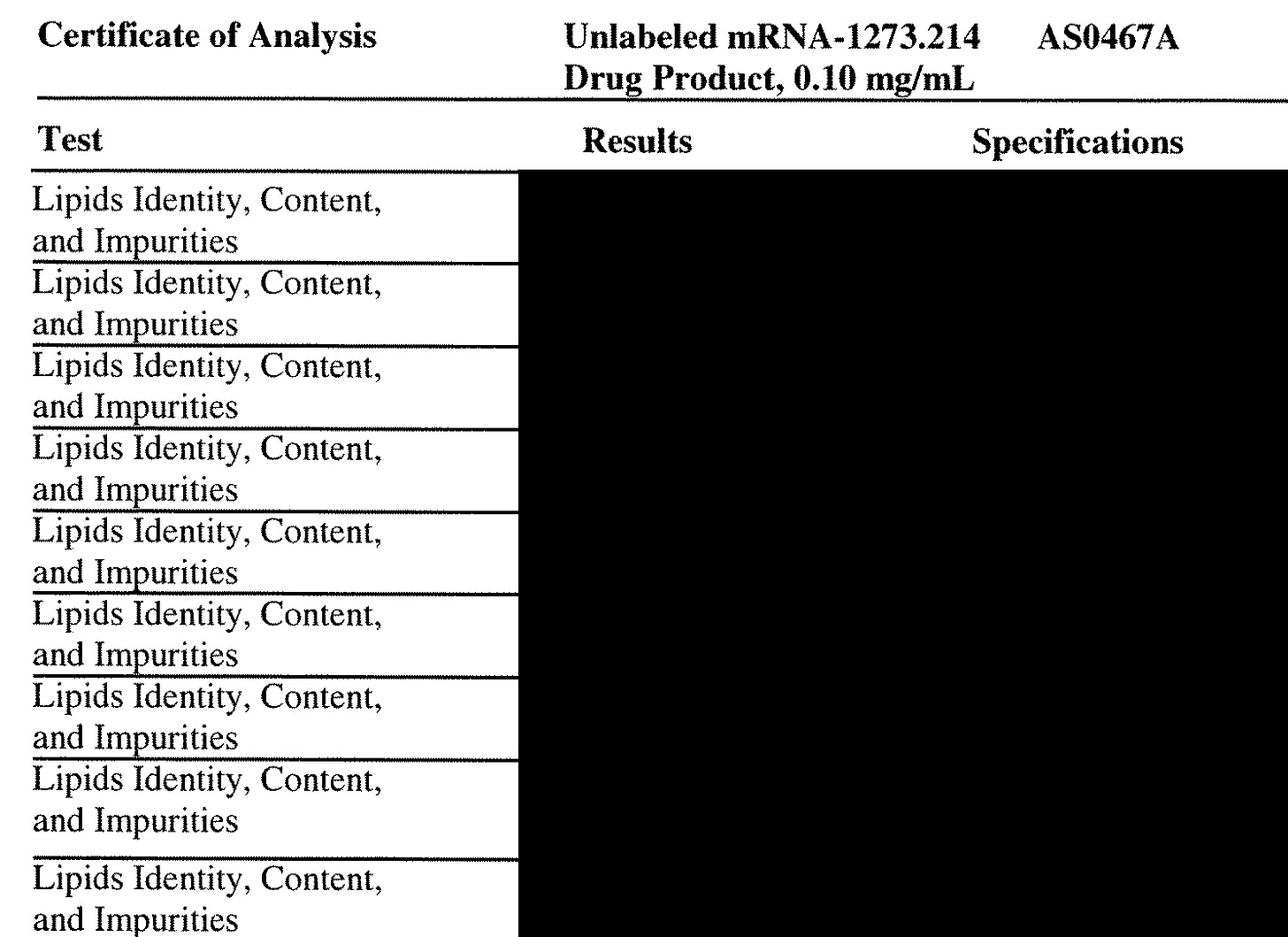
Well, there are at least 10 of them judging by this for Lot AS0467A which is the bivalent BA.1 version (9 on this page and one on the page above this). HOLY TOLEDO. What are these lipid impurities and why is Moderna measuring them and not Pfizer? Even for the XBB vaccines?
4. Lipid Impurities
Well, it appears Moderna is much more concerned with lipid impurities than Pfizer. And this is pretty interesting since Moderna discovered these lipid adducts which I cover HERE.
But what are these impurities? What are they doing? If there are RNA-lipid adducts there must be DNA-lipid adducts as well.
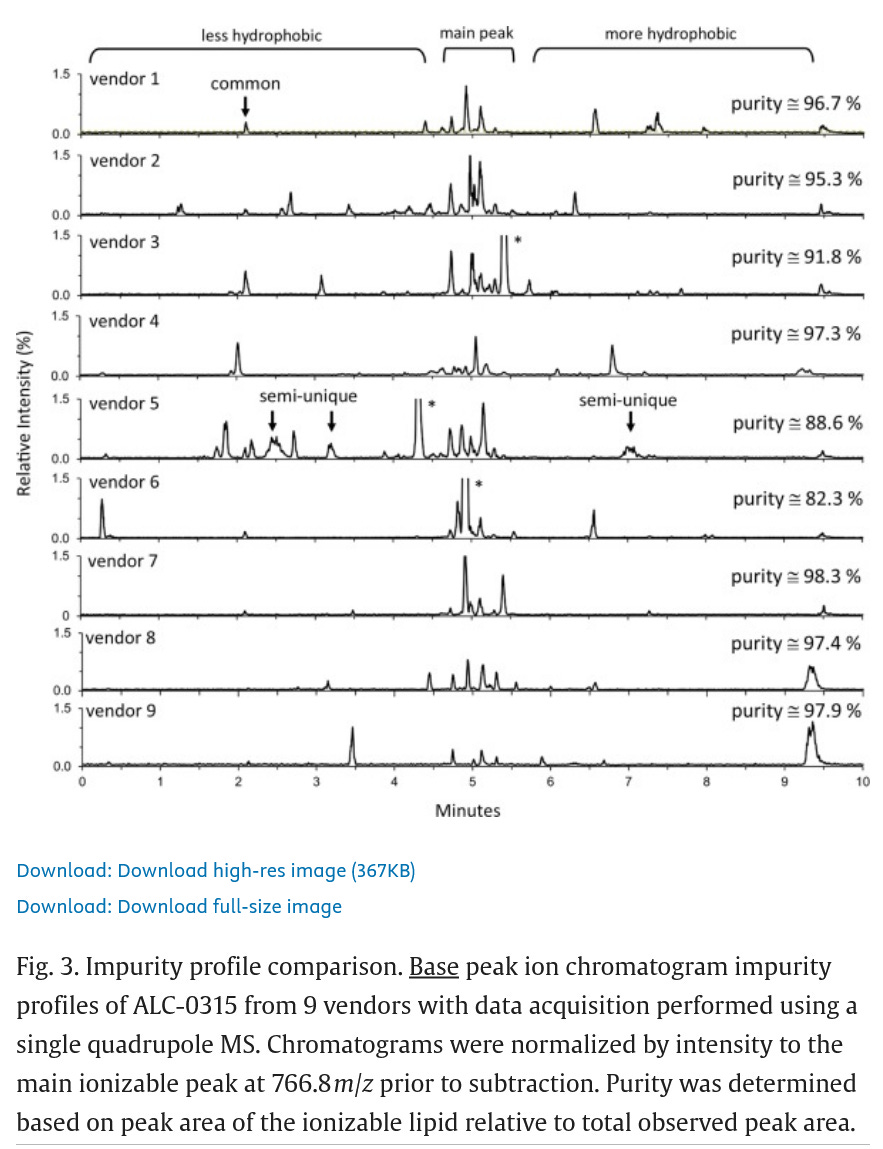
Someone measured the impurities of ALC-0159 from different vendors. Guess you would want Vendor 7. This is just the impurities of the intermediate forms of ALC-0315.
So why are the regulators allowing different attributes to be tested? Is Pfizer’s lipids “cleaner” than Moderna’s? Are they THAT different?
Summary
Available data on lot testing of mRNA vaccines held by Health Canada is restricted to the drug product only. No DNA, no dsRNA
Pfizer changed the analytical method for “identity of RNA” for the XBB vaccines; reason is unclear. But RNA content continues to be measured using fluorometry. Does anyone have any ideas?
Moderna measures 10 different lipid impurities, Pfizer does not (or does not report them). Are the lipids from Moderna significantly different than that of Pfizers??
The analytical methods for Moderna’s lots are unknown but are likely to differ from Pfizer’s
When will the USP finish their compendial standards???? The second draft was April 2023.
That is all the information I could get from this ATIP.
Thank you for reading and pray the rosary.












Known significant impurities in Tromethamine that can potentially cause health problems include:
2-amino-1,3-propanediol (APD)
2-amino-2-methyl-1,3-propanediol (AMPD)
2-(N-methylamino)-1,3-propanediol (MMAPD)
2-(N-methylamino)-2-hydroxymethyl-1,3-propanediol (MMTA)
2-(N,N-dimethylamino)-2-hydroxymethyl-1,3-propanediol (DMTA).
https://geoffpain.substack.com/p/relative-lethality-of-covid-19-vaccines
Lipid impurities for Moderna include Endotoxin in Cholesterol
https://geoffpain.substack.com/p/moderna-ordered-to-get-its-endotoxin
and
Lipid manufacturer published disclaimer
https://geoffpain.substack.com/p/lnps-contaminated-with-endotoxin