Components of the LNP/mRNA Particles
Here is what you are usually shown regarding the components and structure of the LNPs/mRNA particles.
It is a lie. A cartoon. This figure is based on the analysis of Onpattro which I discussed in my previous substack on The Biodistribution of Lipids Part 3. And it is wrong for Onpattro as well.
First however, we need to be clear on terminology regarding lipid based nanoparticles. Lipid nanoparticles are not the same in structure as say pegylated liposomes. Pegylated liposomes have been used to treat patients for almost 20 years now. The different kinds of lipid containing nanoparticles include:
liposomes (used in drugs like Doxil and in cosmetics)
lipoplexes
SPLP (stabilized plasmid-lipid particles, whatever they are I dont know either)
LNPs
Here is the accepted structures of these nanoparticles. Though the rendering of the LNP in this figure is likely wrong as well. So if you thought the mRNA was in a ball in the middle of a lipid bilayer….well that is another lie.
Fig. 5. Simplistic illustration of some of the most tested lipid-based nucleic acid delivery systems in the order of their invention (indicated by the time arrow), starting with liposomes, lipoplexes, SPLPs and finally the LNPs. All structures are proposed structures and can vary depending on lipid composition, nucleic acid cargo and preparation method. The illustration also highlights the trend of decreasing mol% of phospholipids and increasing mol% of cationic or ionizable lipids, introduced to facilitate active nucleic acid encapsulation, which culminates with LNPs containing typically 10 mol% phospholipids and 50 mol% ionizable lipids.
What is the structure of the current LNPs in the mRNA vaccines?
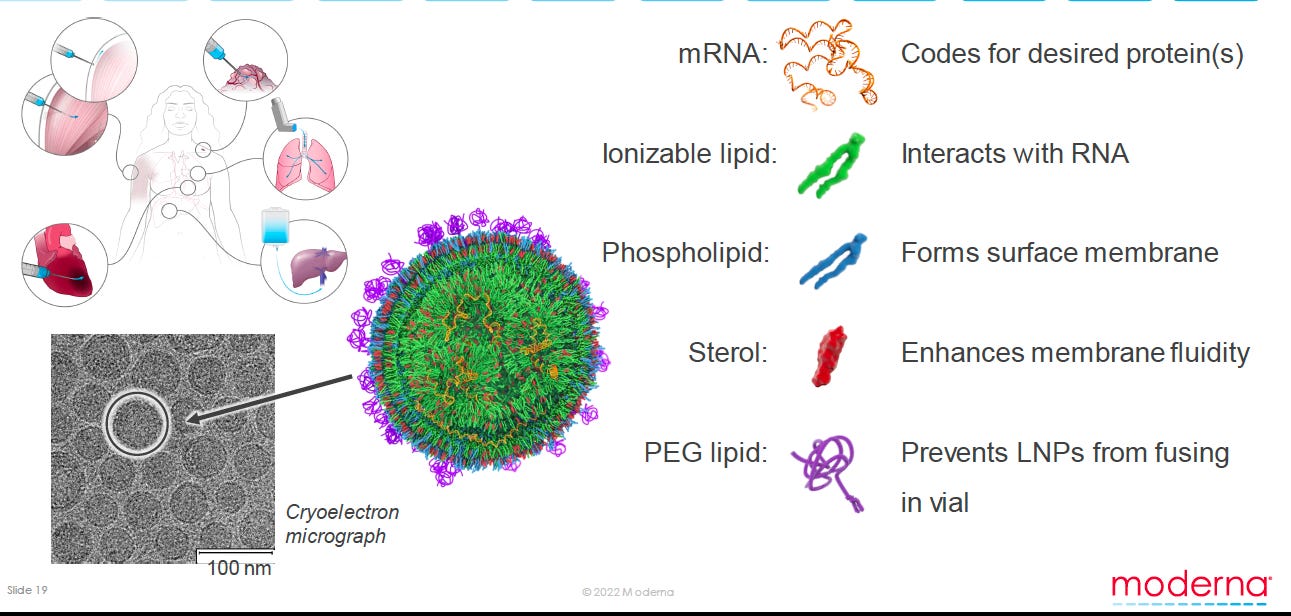
Well I can tell you NO ONE KNOWs. Not even Moderna. Here is a slide from their Moderna Science Day 2022 that tells you what they believe how their LNPs are structured.
They are matching what they see on cry-electron micrograph to a biological rendering of the LNPs. Here they propose a mostly amorphous mass with very little water or spaces. Compare to the proposed rendering above (ie labeled Figure 5)
In addition, changing
the proportion of the 4 lipids making the LNPs
from one ionized lipid to another, or another cholesterol analogue
the mixing, buffers and process for making the LNPs
changes how the LNPs are structured. For example, those LNPs look pretty different from each other
What does CryoEM tell us about LNP structure?
This is where it gets very interesting. A diversity of CryoEM structures have been reported.
The Current Models May Not be Applicable
What was shown for Onpattro regarding structure (and I would say transfection and release on the mRNA in the cytosol) may not be appropriate for the Pfizer vaccine.
Which of these cartoons represent Pfizer’s LNPs? Anyone? Well the authors of the linked paper suggest J, K or L. Who actually knows?
They also compare the Pfizer vaccine thawed and diluted to a real liposomal drug that I have dispensed and used in chemotherapy clinics, liposomal doxorubicin using cryoEM. Notice how different they are? Oh and look at the size range, and in (C) you see BLEBS! The literature is very unclear whether blebs are a good thing (increased ability to empty their cargo into the cytoplasm) or a bad thing (ready to burst on any shearing like being injected in the body).
This paper is great because they show using cryoEM what the Pfizer vaccine (purple tops) looks like after being stored in the fridge for 7 days. Is that mRNA sneaking its way OUT OF THE LNP in Figure C???
So at nanoscale level (best seen with cryoEM and not under a microscope) there is
loss of encapsulation (mRNA escaping)
aggregation (clumping of the LNPs
size increase
sub-visible particles (they can get so big instead of nano sized they are micron sized!!!) (see also my substack on Particulates in Pfizer). And micron-sized pegylated particles are particularly toxic.
Critical Quality Attributes (CQA): LNP size and LNP Polydispersity
As part of the drug product lot testing, there are these 2 CQAs that must be measured
LNP size since size affects biodistribution, and transfection thus affecting safety and efficacy (i.e. the AVERAGE SIZE)
LNP polydispersity is a measure of homogeneity (are they all the same LNPs or are some empty? some with blebs? some with mRNA oozing out?, aggregates etc etc) which impact safety and efficacy.
Currently, the how to measure this is something called Dynamic Light Scattering (DLS). Only DLS is good for telling you if they are a there (the lower the number the more they are like spheres) BUT NOT WHAT THEY LOOK LIKE INSIDE OR THE SIZE DISTRIBUTION. So you can easily have an average size of 120nm (good) but it could be composed of a binomial distribution, lots of little 40-60nm particles and then a few in the 500nm range (not good, you want all under 200nm). And you really dont know how many are empty.
So at the present time, NO ONE KNOWS HOW TO BEST MEAURE THESE LNPs, let alone TELL WHAT IS INSIDE THEM.
I think this is why Moderna has partnered with McGill as reported here
McGill University and Moderna to expand collaborations with new projects in Lipid Nanoparticle research
Two projects will analyze characteristics of lipid nanoparticles as well as naturally occurring particles known as Extracellular Vesicles (EVs)a. Dr. David Juncker, is undertaking a study to characterize LNPs so that their specific size and payload distribution may be better understood since current techniques (like DLS) cannot simultaneously quantify the size and payload of individual LNPs, and thus often only averages are measured that mask individual variation and mask the relationship between size and payload.
Full steam ahead with the LNPs. They’re going to FIX this even if it wastes tons of $$.
Summary
the actual internal structure of an LNP with mRNA is unknown even after 4 years
there are several competing hypothesis and several types of LNPs seen under cryoEM even within the same vial
stuff is happening to the LNPs at nanoscale that is not well understood
what to measure and how to measure for safety and efficacy is still being worked out
SO WHY ARE OUR REGULATORS SAYING THE mRNA PRODUCTS ARE WELL CHARACTERIZED AND ARE SAFE AND EFFECTIVE WHEN EVEN THE MANUFACTURERS ADMIT THEY DONT KNOW WHAT IS GOING ON?
KNOWINGLY, KNOWINGLY, KNOWINGLY
Thank you for reading and pray the rosary.











Oswald Ripening
Nice
Outstanding Canning
Outstanding