The Measurement Problem of the mRNA jabs: Part 2
Part 2: the modRNA impurities with emphasis on dsRNA: a bonanza for analytical and device manufacturers
So in Part 1 we learned of what CQAs are and that they include a specific analytical method.
The US Pharmacopoeia has set out to devise compendial standards and help manufacturers with CQAs. Background on compendial.
Compendial, what's that?
Much of the stuff I have been looking at regarding manufacturing defects and linking to adverse effects are related to the analytical methods used to determine quality and purity. We reviewed the critical quality attributes in my previous post, now we we look at the analytical methods itself.
So things have been moving along in the analytical land for mRNA vaccines. Lots of money is being spent on figuring out how to make them cleaner and how to analyze them. Serious money.
modRNA Content
This is easy. You just need to measure how much modRNA is there, usually as a concentration ie mg/mL. The analytical test used for that in Process 1 and Process 2 was fluorometry. That measures ALL mRNA, all the bits and pieces that occur because of the in vitro transcription. So not all that is measured is actually going to be able to make spike protein. But is fluorometry going to be the compendial standard?
Uh, no.
USP is proposing
qPCR
ddPCR
UV fluometry
So that means mRNA and DNA template will have the same analytical method (since residual DNA will still be using qPCR). This should alleviate some of the “gaming of the system” by using analytical methods which are not really comparable that Kevin McKernan talks about here. They use Fluorometry to measure the RNA levels and qPCR to measure the DNA levels!
Why wasn’t that done before????
modRNA Intactness
So this goes back to the fact that producing modRNA via in vitro transcription, especially at scale has problems. Intactness is measured pretty easily with CGE. Here are some examples of what this looks like.
The bottom B panel is blown up to show all the fragmented modRNA. As most know, the original (and I believe ongoing) specifications is that the percent intactness is >55%. I am pretty sure it has improved but seriously, how could this level of bad manufacturing be approved? There are sometimes bumps to the right of the main peak, those are the lipid adducts which I talk about in previous articles. There are ongoing analysis to see exactly what is under those bumps and if they are hiding other impurities, say like aggregated mRNA, stuff like that. Here’s a review to any analytical chemistry geeks if you’re interested. Comprehensive impurity profiling of mRNA
What is interesting is that unless the intactness was >60%, then there was very little actual spike protein being made.(see figure 1C of this paper ) using the best potency test for mRNA vaccines to date. There was no effect of temperature; you need at least 60% intactness to get protein expression. HMMM. Maybe it was a good thing Process 2 lots had such poor intactness……
modRNA Process-Related Impurities
OK, here is where things get really interesting. At the approval of the vaccines, there was only 2 process-related impurities that were measured.
the residual DNA template
double-stranded RNA
I won’t talk about the DNA template and issues with the analytical methods as I am sure most of my readers are familiar with this. I WILL take about the dsRNA and why it occurs, and how to measure it. But FIRST:
NEW Process-Related Impurities
I was very pleased to see that USP and actually the industry itself (well maybe not Pfizer or Moderna, but others who want to make “clean” mRNA therapeutics) added these other impurities to the list that must be analyzed and kept as low as possible.
the T7 polymerase itself.
the T7 polymerase is a bacterial enzyme. There is some left over after it does its thing making the DNA template into modRNA in the in vitro process I talked about in Part 1.
what does a bacterial enzyme or protein do inside the cell? It may stimulate the immune system to cause adverse events? Decrease mRNA translation to protein (we cant have that)? I am not sure it is yet routinely measured.
AND there is not standards for the purity and activity of the T7 polymerase itself. How potent is it? How much endotoxin? Etc Etc
An important point. ALL THE CHEMICALS, REAGENTS, STARTING MATERIALS FROM THE DNA PLASMID TO THE BUFFERS AND LNPS HAVE TO BE PURE AS POSSIBLE SINCE THIS IS A TRANSFECTING AGENT.
unincorporated nucleotides
OK, this one I anticipated and am glad it is being considered. What happens if you’ve got extra synthetically made nucleotides in the cell? Especially N-1-methylpseudouridine? Does the body use these unincorporated nucleotides in other mRNAs? What would be the effect if a normal protein designed to be short acting in your body is now being coded with N-1-methylpseu and the proteins’ duration of action is longer? Is it even possible?
what happens if these nucelotides get oxidized because of the conditions within the LNP? Supposedly this is associated with neurogenerative disease.
Ummmmm this seems kind of important, no? I have not seen too much on this in the literature. Maybe it’s not such a big deal, and the levels are quite low, but I need empiric evidence.
residual solvents
this is SOP and there are solid compendial standards on this. Pharma knows how to get rid of most residual solvents to a very low amount
the issue here, is again, that these products are transfecting residual solvents into the cell. Maybe the limits need to be revised?
endotoxin
so far, it has not been an issue using the LAL compendial standard from a regulatory point of view.
But are those standards appropriate? For a transfecting agent? Also, endotoxin on the outside of the LNPs are not measured well. I brought that up to USP, and they took this seriously. I hope new analytical techniques will be developed that can really measure endotoxin levels accurately in these jabs. Geoff Pain is the man to read for these issues.
dsRNA process-related impurities
dsRNA impurities is one of the fatal flaws of the mRNA vaccines, and Pharma knows it. If you read the trade literature, residual DNA is hardly ever mentioned, it is all about dsRNA.
AND, THE MONEY POURING IN TO TEST THIS IMPURITY IS HUGE.
Why all the fuss?
Residual dsRNA is a major contaminant of the modRNA drug substance secondary to the IVT process. dsRNA by-products such as runoff transcripts or an antisense RNA molecule similar in size to the desired mRNA can occur but require various analytical procedures for determining purity and quality. The length of the dsRNA such as looped back RNA has been linked to the severity of adverse events.
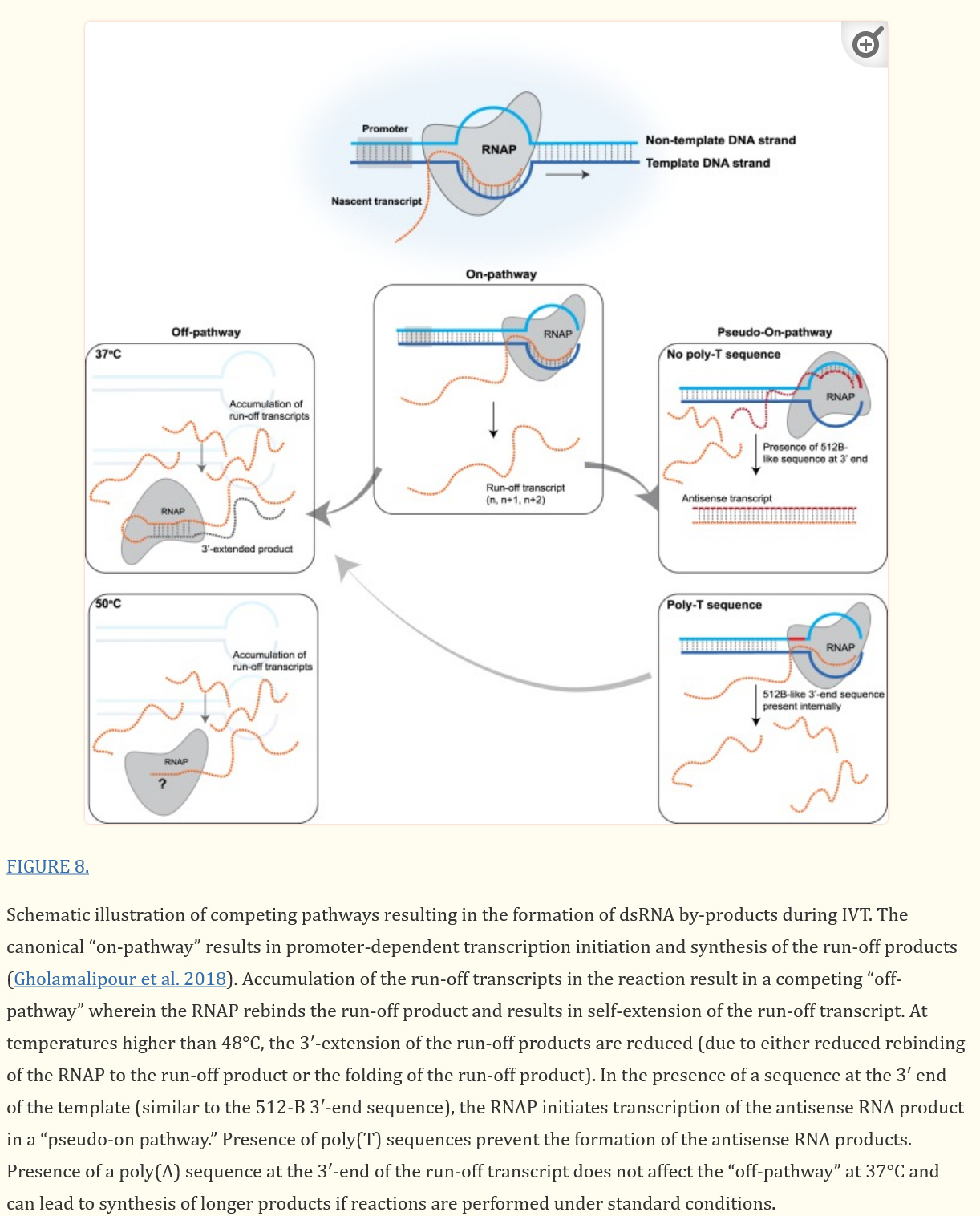
Here is what happens during the in vitro transcription. These authors are determining if temperature of the IVT process makes a difference. The RNAP here is the T7 polymerase used for the mRNA vaccine production.
There are all kinds of dsRNA that can occur. You know it is a problem when
Moderna patents a new T7 polymerase Dousis et al
BioNTech patents a new method of IVT that results in very low levels of dsRNA
Or invent a new 5’ cap like the Japenese did PureCap technique, for producing mRNA vaccines with exceptional purity and high activity
there are all kinds of purifying techniques
WHY IS NO ONE TALKING ABOUT THIS?
My very first substack was on the dsRNA contamination and what was doing about it and whether this would be considered an adjuvant. And how immunogenic it is and how it may be related to myocarditis. Moderna has much more dsRNA in it than Pfizer for various reasons. Over a year ago I was musing on this.
How to measure these dsRNA products
So now we are getting serious about dsRNA. Some of these molecules are the SAME SIZE as the ssRNA or mRNA molecule you want. So it is hidden, so to speak.
Pfizer, and I think Moderna used immunoblot to test dsRNA as it is considered a standard techniques. Immunoblot is SEMI-quantitative. Good enough for a lab but not for a pharmaceutical product.
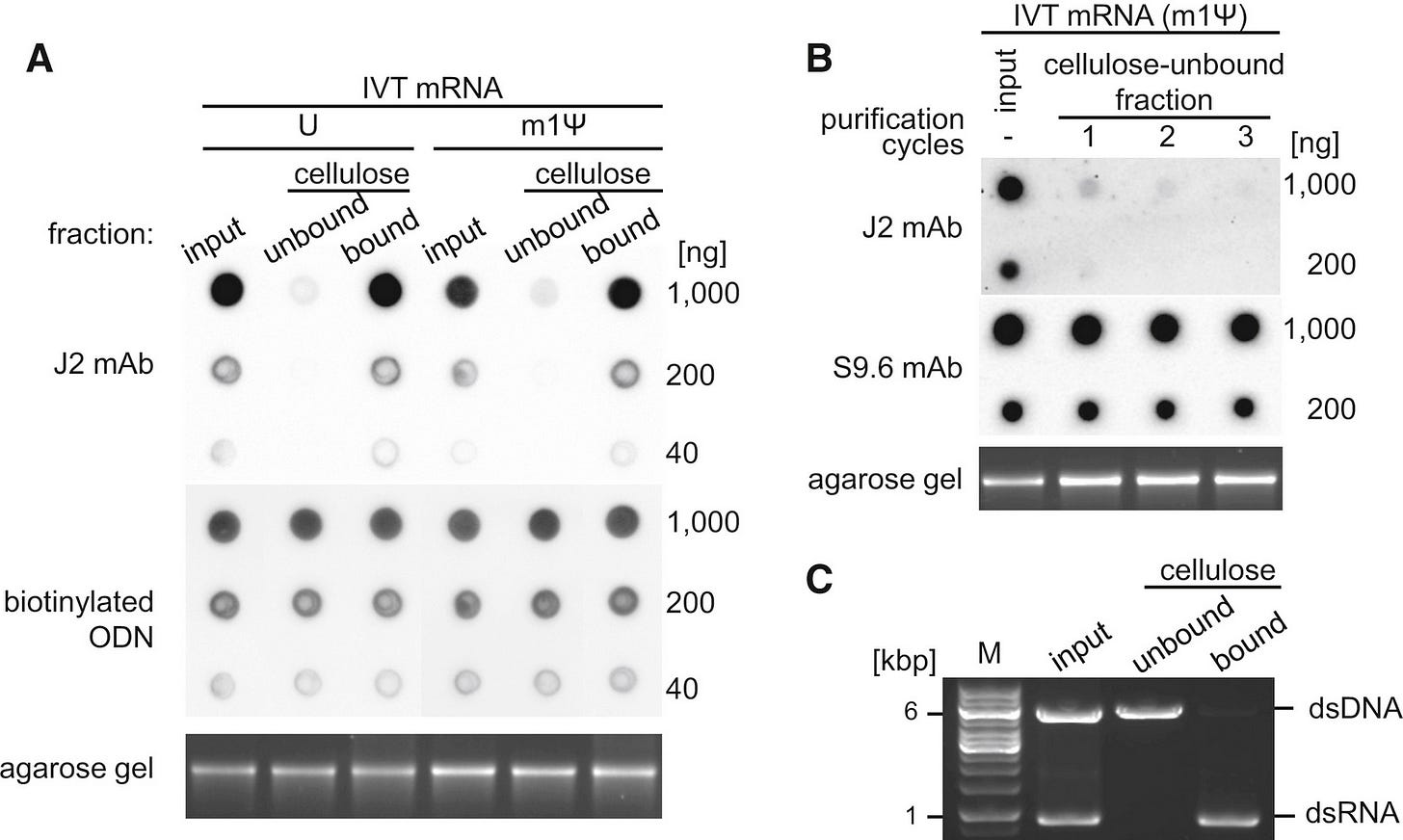
Here is BionTech using their patented method of getting rid of dsRNA using cellulose and ethanol. Immunoblot is an antibody based test using J2 mAb. See in panel B? dsRNA is all gone? Right?
Not so fast.
Immunoblot does not measure this loop back dsRNA and the longer lengths of dsRNA may be associated with more severe adverse events especially cognitive dysfunction in aged mice. Is anyone talking about this??
The USP has added ELISA as a more sensitive method but these loop-backed dsRNA are STILL difficult to quantify.
If the 3’-end of the runoff transcript has sufficient complementarity (in cis), it will fold back and result in extension of the runoff transcript. In the second mechanism, the formation of dsRNA byproducts results from the RNAP using the nontemple strand, resulting in an RNA molecule that is complementary to the runoff product but synthesized in a promoter-independent manner. Because the size of the antisense molecule will be similar to the size of the runoff product, it cannot be distinguished by denaturing gel electrophoresis
This has lead to an explosion of various methods by companies hoping to cash in on the mRNA vaccine bonanza
Just some examples
And most importantly, maybe we should measure dsRNA in the cells being transfected so we know what the dsRNA is doing in there?
What about the new self-amplifying RNA vaccines?
Ohhh, no worries you silly goose. It is a self-adjuvanting vaccine. Ummmm isnt that true about normal mRNA vaccines and we find that it is hard to measure and is associated with lots of adverse events? What are they thinking? Our own Canadian Anna Blakney is a fan of saRNA vaccines.
saRNA is considered to be self-adjuvanting due to the dsRNA structures, replicon intermediates and other motifs that are sensed intracellularly [38]. However, recent studies have investigated the role of both the delivery vehicle and molecular components in adjuvanting saRNA vaccines. Blakney et al. observed that the adjuvancy of incorporating 3M-052, a TLR 7/8 agonist, into lipoplexes was eclipsed by the self-adjuvanting effects of saRNA. Démoulins et al. found that incorporating Pam3Cys-SK4 (P3C), a bacterial lipoprotein, promoted saRNA internalization by DCs in vitro but did not enhance humoral or cellular immunogenicity in vivo
So including a TLR agonist is STILL not as “adjuvanty” as the saRNA itself.
What are they thinking? That giving a little itsy bitsy dose will do it? When there really is no “dose” in mRNA vaccines.
Gah.
Summary
OK I was starting to get into a real ranting mood with the saRNA vaccines so I need to calm down
analytical methods is a big black hole for mRNA vaccines. If you don’t know WHAT to measure, WHAT is the “specifications” or appropriate limits and HOW to measure it, we are in trouble. So many unknown unknowns.
This new platform technology has still a long way to go regarding manufacturing quality and safety. Certain things have definitely improved, such as measuring mRNA integrity and better specifications for that.
I don’t think we have gotten our heads around transfection, and how it bypasses all our integral cell membrane and all that entails.
dsRNA is THE MAJOR contaminant in the vaccines imho from the IVT, combined with the errors in transcription represent fundamental flaws in this technology that I am not sure can be fixed. Just covered up or made as low as possible.
dsRNA requires MULTIPLE analytical techniques to measure it. Because you don’t even know it is there. Also how much should be allowed? 1.5% or 0.5% or less? Have we done the studies?
NEW critical quality attributes for the drug substance has been added. This is welcome. BUT are there more to come?
These researchers etc need to study physiology and pharmacology and get a good idea of the variability and infinite complexity among humans and that we are not machines to be tinkered with.
Thanks for reading my rant.
Oh and pray the rosary.






"WHY IS NO ONE TALKING ABOUT THIS?" A substantial part of my book deals with dsRNAs, how it can be generated by T7pol, their adverse potentials, and, analogously, shortRNAs. It's been over 1.5 years that it has been published. Even though it's a Springer book, people do not seem to want to read it or care about these issues.
"These researchers etc need to study physiology and pharmacology and get a good idea of the variability and infinite complexity among humans and that we are not machines to be tinkered with." In all honesty the detail in most of your article went over my head, in this sentence you have expressed what I was trying to bring to the front of my mind. The human race is infinitely complex and we are not machines to be tinkered with, not one of these injections should have been given to any human. The researchers and the pharmaceutical companies were so full of hubris that they gave no respect or recognition to the complexity of the beings they experimented with and went ahead with it because they could and no one was there to stop them.