LNPs as Supramolecular Assemblies: Changing the Object of Analysis
Why lipid nanoparticles must be understood as assembled drug entities
Introduction
Much of the current confusion around mRNA vaccines does not stem from lack of intelligence, bad faith, or even missing data. In my opinion, it comes from a category error: we are analyzing the wrong OBJECT.
My collaborator Falko, better known as Genervter Bürger, had a long debate with a regulatory and research pharmacist Hans Joachim Kremer on BittelTV, an independent German media outlet. You can see the debate here.1 The debate is in German but I used the autotranslate. What struck me quite forcefully is how HaJo (as he is colloquially known) looks so lost at what Falko is saying. It is as if they are talking past each other.
I think the disconnect comes from the fact that most clinicians, pharmacists, and regulators are trained to analyze individual substances, that is discrete molecules with definable structures, ADME pharmacokinetics (administration, distribution, metabolism, excretion), and dose–response relationships. But LNPs are not administered as isolated substances. They are administered as an assembled systems. This is a bit of a paradigm leap, so it took me some time as well. If you go through my old posts, you can see my understanding building from individual substances to assembled LNPs, especially after my “The Strange World of Nano” post.2
This distinction matters, because biology interacts with assemblies, not individual ingredient lists.
LNP Supramolecular Assembly: the core claim
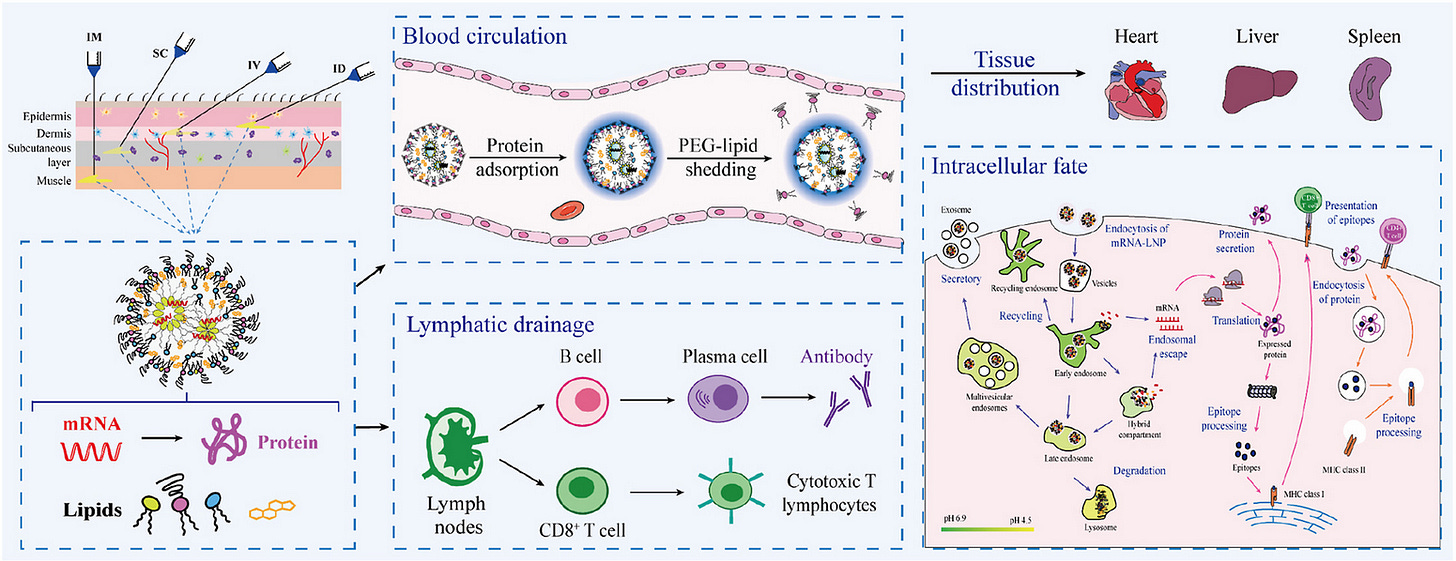
Although the therapeutic intent of the modRNA vaccines resides in the mRNA, the actual FIRST “drug” the body encounters is the assembled lipid nanoparticle.
Everything that follows, specifically, biodistribution, early adverse reactions, immune engagement, tissue exposure is affected by that assembled entity, long before any protein is translated. This is what I have been calling the pharmacological phase, from injection until the mRNA emerges in the cytoplasm. This was first articulated by Naasani here.3
This reflects my ongoing understanding of this platform, where I and others proposed that the mRNA is the pro-drug which then gets translated to the active substance, the spike protein. I had not really understood the LNPs at that point, but as I started delving into their manufacturing 2 years ago, I’ve come to realize they are the actual driver of this platform and the “first drug” in this multicomponent vaccine platform, not the modRNA.
What is meant by an LNP assembly?
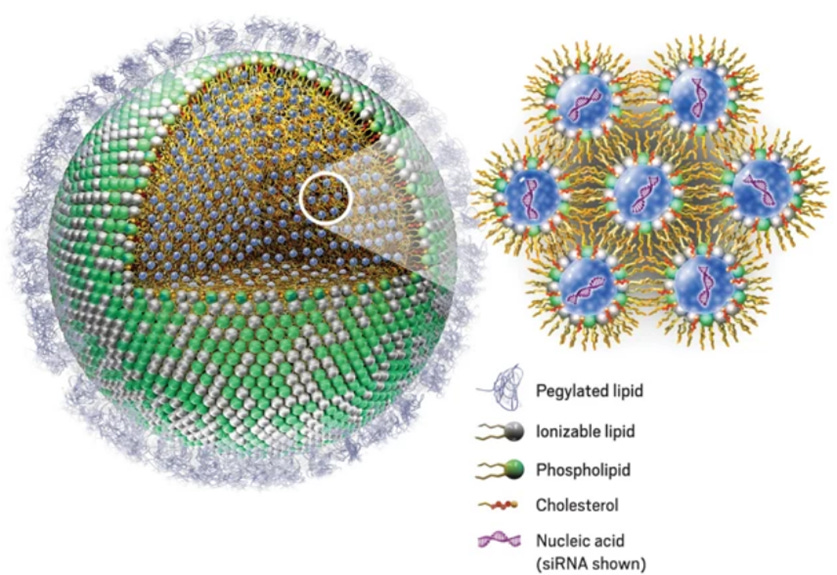
A LNP is a supramolecular assembly of amphiphilic* molecules that self-organize into nanoscale structures capable of encapsulating nucleic acids (e.g., modRNA or siRNA). They self-assemble AROUND the modRNA.
*Amhiphilic means contains a water attracting (hydrophilic or polar) region and a water-repelling (hydrophobic, nonpolar) region within the SAME molecule or structure.
But what do I mean by a SUPRAMOLECULAR assembly?
Structural Aspects
Ionizable lipid: complexes with the negatively charged nucleic acids through electrostatic attraction, forming the core.
Cholesterol: stabilizes the membrane and modulates fluidity using van der Waals-based forces
Phospholipid: contributes to bilayer or micelle-like domains through hydrophobic interactions.
PEG-lipid: provides colloidal stability via steric repulsion and controls aggregation.
These components collectively form a self-assembled, thermodynamically stable system, HOWEVER, it is metastable. That is, it can decompose or reorganize under certain conditions (pH shift, dilution, or temperature change), unlike true covalently bound nanoparticles. Thus they are dynamic. This is why they need a strict cold chain, freezing etc.
Key Properties of LNPs as Supramolecular Systems
Dynamic composition – Lipid molecules can exchange or rearrange, enabling fusion, cargo release, and degradation.
Adaptive structure – The assembly responds to environmental changes (e.g., endosomal pH triggers disassembly for mRNA release). This aspect is critical for its function.
Reversibility – Formation and breakdown rely on reversible, non-covalent interactions. HOWEVER, there ARE covalent bonds formed in small amounts, the so-called lipid adducts as a result of oxidation. But ideally there should be none because you want the LNPs to disassemble inside the cell. Because the LNPs are supposed to be fully reversible the discovery of adducts was distressing. See my previous posts here4 and here.5
Emergent function – The collective behavior (e.g., transfection efficiency, biodistribution) emerges from the whole system rather than any single lipid type.
It is the EMERGENT function that we need to think about with LNPs. It is not just a bunch of molecules assembling, the resulting nanostructure has emergent function which is often unknown, and UNPREDICTABLE. That is, you cant always figure out the emergent function a priori, which is why manufacturing LNPs is difficult, and there is a LOT of trial and error. This also means manufacturing pharmaceutical grade consistent batches with the same emergent functions is well nigh impossible, imho.
If you are interested here is an article on supramolecular chemistry,6 and here is another on LNPs as supramolecular assemblies.7
Supramolecular assembly versus single lipids
Once you understand this supramolecular concept, then you are no longer dealing with a classical small-molecule drug, or a collection of small-molecular drugs. There are no individual molecules diffusing randomly in a solution. There are no receptor-ligand interactions. Instead you are dealing with a:
A nanoscale patch of membrane-active charge density composed of tens of thousands of amphiphiles acting cooperatively.
One way to move away from thinking about individual lipid molecules and toward the particle as the relevant pharmacological object, it helps to do a simple order-of-magnitude calculation. A typical mRNA vaccine lipid nanoparticle has a diameter of roughly 80–100 nm; taking 100 nm as a conservative estimate gives a surface area of about 31,000 nm². Individual lipid headgroups occupy on the order of 0.5–0.7 nm², meaning that the outer leaflet (shell) of a single LNP contains on the order of 50,000 molecules. Holy Toledo.
Given that ionizable lipids typically comprise ~40–50 mol% of the formulation, this corresponds to roughly 20-25,000 ionizable lipids presented at the particle surface at any given moment. What cells therefore encounter is not an isolated lipid molecule, but an interface composed of tens of thousands of amphiphilic lipids acting cooperatively. This is at a scale at which emergent, supramolecular behavior is not only plausible, but expected. And as a result, this system follows threshold physics, or is more physical chemistry and pharmacology rubrics than biolological type interactions.
This is not a dose–response problem at the level of individual molecules, but a threshold problem at the level of assemblies.
Once the relevant unit of interaction is recognized as a cooperative supramolecular interface rather than an isolated lipid molecule, it becomes clear why classical ligand–receptor intuition fails and why non-linear, context-dependent biological responses emerge with this platform. The governing principles are less those of classical ligand–receptor biology and more those of physical chemistry and pharmacology.
Here is a representation of an LNP with the ionizable lipid making up the shell to give you an idea. (along with the helper lipid in green). Each grey sphere on the LNP shell represents the proton head of the ionizable lipid.
What are the clinical implications?
To understand the clinical implications of the modRNA–LNP platform, the most important question is not how long lipid nanoparticles persist, nor whether individual components are inherently toxic. The key question is more basic: who is doing the assembling?
Who is doing the assembling?
Phase 1: Manufacturing assembly
LNPs are first created by human-directed supramolecular assembly:
Microfluidic mixing
pH-driven electrostatic condensation
PEGylated surface stabilization
A discrete, nanoscale object is manufactured before injection into the body.
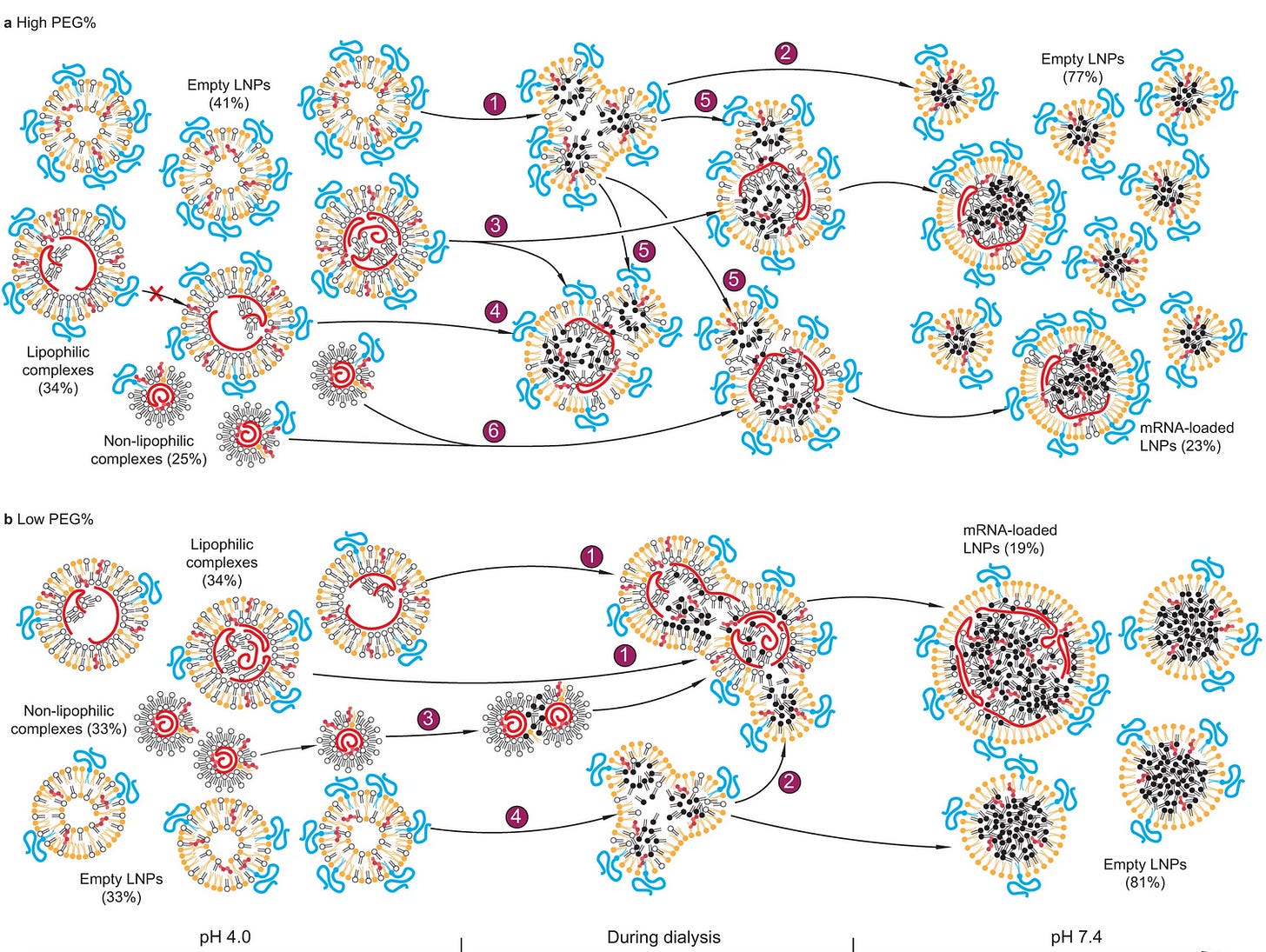
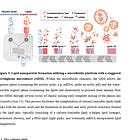
Here is a figure of what Li et al8 thinks is happening as the LNP is made and in this case, encapsulates siRNA, then dialyzed. This is to show the self assembly. His method measured that about 80% of the LNPs are empty, but others disagree (estimates range from 13-80%, average about 30-35% of LNPs are empty).
High PEG: Each number label represents a populational behavior during dialysis: 1, splitting of empty LNPs; 2, stabilization of empty LNPs; 3, splitting of lipophilic complexes with an initially high mRNA payload; 4, remaining a same mRNA payload for lipophilic complexes with an initially low or intermediate payload; 5, merge of empty LNPs with mRNA complexes; 6, merge of non-lipophilic complexes. The cross mark represents the finding that the mRNA payload of lipophilic complexes does not increase during dialysis due to lack of merging under this condition. Low PEG: The labels are: 1, merge between lipophilic complexes; 2, merge of empty LNPs with mRNA complexes; 3, merge of non-lipophilic complexes; 4, splitting of empty LNPs.
Phase 2: Biological re-assembly
Once injected, the BODY ITSELF becomes an assembler. This is crucial to understand.
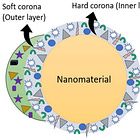
The Protein corona is formed within seconds. see my post here
Complement binding and CARPA occurs. see my post here
Lipid exchange with plasma lipoproteins forming “ghost particles” see my post
Cell membrane insertion and remodeling (see this interesting preprint9)
Endosomal lipid mixing
No new covalent bonds are required for any of this. Persistence here is functional, not molecular. This is important to remember and understand as I believe it may explain many adverse effects.
What the body actually “sees”
From a biological perspective, the body does not encounter:
free mRNA
free ionizable lipid
free PEG
It encounters a slightly charged, PEG-decorated, membrane-active nanoparticle with its own biological identity. Cullis et al really make this point10, and it is the reason for the lack of in vivo to in vitro correlation.
As a result, the immune system, complement cascade, endothelium, and cell membranes respond to:
surface geometry
charge dynamics
curvature
LNPs persistence in circulation through biological remodelling, though they many not be the exact LNPs first injected from the syringe. That is, the immune system respond to the supramolecular assembly of the LNPs, not their individual components. And the lipids themselves can persist in cells or in lymph/plasma through biological remodelling or reassembly via our own lipid trafficking pathways.
Why this supramolecular framing of the LNPs matters clinically
When the assembled entity or the nanoparticle nature of the LNPs are ignored, lots of stuff are missed or cannot be explained with the model that was given to clinicians. Therefore they are left with:
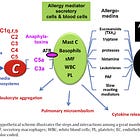
unexplained immediate reactions (are they ALL allergies?)
heterogeneous tissue effects
timing mismatches
inappropriate AE labeling (ie CARPA as anxiety, stroke, clots etc)
What emerges is a modified biological context: altered membranes, perturbed trafficking pathways, activated innate immune signaling, and vesicular systems carrying non-native lipid and RNA components. These system-level changes can persist long after the original formulation has dispersed as we discussed in our preprint.
When the assembled entity of the LNP is acknowledged, these observations become mechanistically coherent.
This article describes the pharmacological phase quite well and is highly recommended.11
Changing the object of analysis to inform clinical assements
So overall, when we change the object of analysis from the mRNA to the LNPs, the analysis appears more like the following:
LEVEL 1: INGREDIENT VIEW: common clinical / regulatory lens
modRNA
ionizable lipid, PEG lipid, helper lipid cholesterol
analyzed separately
assembly ignored
▼
LEVEL 2: ASSEMBLED ENTITY: pharmacological reality
70-100 nm particle
PEGylated surface (stealth but immunologically active)
dynamic surface charge (neutral to cationic depending on environment and biocorona)
high curvature lipid surface (shape)
metastable structure, time-dependent dissassembly
this is what enters the body, blood, plasma, lymph
biological identity and biological interactions
▼
LEVEL 3: SYSTEM RESPONSE: what clinicians observe
OUTSIDE THE CELL
transient LNP assemblies (LNPs can dissassemble in plasma, or form ghost particles)
lipid exchange with plasma membranes (e.g.remove or swap out cholesterol from lipid rafts etc)
complement activation (CARPA), pattern recognition, membrane effects
initiation of signaling without entry
short lived supramolecular assemblies but long lived signaling
INSIDE THE CELL
disassembly of LNPs
endosomal sorting and membrane recycling
lipid-modified endosomes / lysosomes
exosome paracrine pathways (and possible “shedding”)
prolonged intracellular consequences without intact LNPs (see our preprint12)
the particle is gone but the individual lipids themselves are not
short-lived assemblies but long-lived cellular remodeling
timing and heterogeneity is important
▼
modRNA release and translation into spike protein
then and only then does the spike protein get translated
mRNA release
Translation of spike
Antigen presentation
Adaptive immune activation
This is not the first drug effect. It is the intended immunoloical effect — but not the earliest. I believe changing the object of analysis from ingredients to a supramolecular assembly resolves many apparent paradoxes.
Timing matters
As analytical methods improved, time-resolved behaviour could be studied.
At time ≈ 0–minutes:
LNP supramolecular assemblies interact with plasma membranes
complement activation
membrane reorganization
lipid exchange (ghost particles)
signaling initiation
At time ≈ minutes–hours:
endocytosis
endosomal stress
lipid redistribution
innate sensing
translation begins locally
However, these processesess are not sequential in a neat chain. They are overlapping and mutually conditioning.
Spike may appear:
while membranes are already perturbed
while signaling is already altered
while trafficking pathways are already affected
So spike expression occurs inside a modified cellular context. I think this is crucial to understand, and this does not reflect those pretty pictures we see which describe a sequential series of actions of how the vaccines were thought to work. How does that change what we think is happening with the spike protein?
At time ≈ hours-days
further biodistribution and recycling
endothelial and tissue exposure
possible sequestration in adipose or other quiescent cells (speculation) via adducts or vescicles, lysosomal storage
further spike protein production at low levels at “off target” sites
I dont know what happens weeks to months to years afterwards, but it is unlikely in this context of supramolecular LNPs for spike production per se to continue constantly. Other processes could explain what we see. HOWEVER, cellular signalling from the LNPs sure could, sequestered LNP assemblies could be disturbed via injury/stress/infection and tiny amounts of spike protein made triggering a boat load of antibodies to form. But this is all speculation on my part and this is not required for the argument above.
Why this is not a semantic argument
In the materials science literature, some LNPs are explicitly termed supramolecular only when host–guest recognition (e.g., cyclodextrin inclusion) is engineered into the structure. But that is a design classification. Doxirubicin is an example of this.13
What is discussed here is a pharmacological classification, since any non-covalent assembly whose biological effects emerge at the level of the assembled system (as is the case with the LNPs) should be analyzed as such.
This is already how clinicians understand liposomes, IV iron complexes, polysorbates, and contrast agents. And note, almost all of these are associated with infusion reactions such as CARPA. Because these are all particles.
One more thing: analytical methods were immature
Originally the analytical methods to measure LNPs were optimized for formulation quality control and not for in vivo dynamics, biological remodeling or for any aspects of time-related behaviours as discussed above. That is because early analytical tools assumed lipid nanoparticles were stable inert particles.
As measurement techniques improved, what became more obvious was a highly dynamic system showing a system that assembles, remodels, and interacts with biology in ways more consistent with supramolecular behavior than with a fixed drug entity. Most of these new analytical methods have exploded in the last 1-2 years. The poor analytical methods is why Pharma could not tell us how many particles per dose, or how many modRNA constructs per LNP, or whether there were aggregates or not.
From a regulatory point of view, not having compendial standards or established analytical methods in a problem, because how do you know how the product works if you haven’t established WHAT to measure, nor HOW to measure it. For the LNPs, given that it was considered an inert excipient, detailed analytical measurements may not have been considered essential. That is no longer tenable, and the analytical chemists know it.
Summary
We need to define what we are looking at, and what the body is reacting to. And that starts with correctly identifying what is administered.
The effects of lipid nanoparticles do not increase linearly with the addition of individual molecules, Instead, they exhibit threshold behavior: below certain local concentrations or surface densities, little happens; above them, qualitatively new behaviours emerge.
Membrane perturbation, curvature stress, complement activation, and endosomal escape are all cooperative, non-linear processes that depend on collective lipid behaviour rather than on specific molecular recognition (ie receptor-ligand interactions). And the LNPs as supramolecular assemblies describes these effects.
The biological responses that follow are downstream of a physicochemical event which is the supramolecular LNP interaction in the body that once initiated, can propagate through biological signaling pathways long after the original assembly has dispersed. So even if the LNPs are “rapidly cleared,” their biological effects may not be.
The lipid nanoparticle is not an inert delivery vehicle. It is the pharmacological entity the body encounters first.
When the LNPs are understood as short-lived supramolecular assemblies governed by physical chemistry and pharmacology, instead of inert carriers, several long-standing puzzles begin to resolve. The timing of adverse events, their heterogeneity, their poor correlation with circulating spike protein levels, and their resistance to conventional dose–response models all become easier to analyze and interpret.
Seeing the LNPs as supramolecular assemblies does not require long-lived intact particles nor does it depend on the persistence of the WHOLE assembled LNP as a stable supramolecular entity. It requires only transient LNP assemblies capable of crossing biological thresholds/membranes and initiating downstream biological effects. That is well within the established domain of pharmacology. Many drugs work in this manner.
In the end, pharmacology (and the physics beneath it) always wins
Thank you for reading. I know this is a heavy and a bit paradigm shifting so please ask questions and I will try to explain or write another post.
And as always, continue to pray the rosary.
https://link.springer.com/article/10.1007/s40262-022-01149-8
https://pmc.ncbi.nlm.nih.gov/articles/PMC12409610/
https://www.sciencedirect.com/science/article/pii/S2590257121000547
https://www.nature.com/articles/s41467-022-33157-4
https://www.biorxiv.org/content/10.1101/2024.11.27.625717v2.full.pdf
https://www.liposomes.ca/publications/2020s/Francia%20et%20al%202020%20-%20The%20Biomolecular%20Corona%20of%20Lipid%20Nanoparticles%20for%20Gene%20Therapy.pdf
https://www.sciencedirect.com/science/article/pii/S2211383525007737
https://www.preprints.org/manuscript/202511.0517
https://pmc.ncbi.nlm.nih.gov/articles/PMC11012056/







They’ll say: Oh, but that’s just the delivery system. Nonsense. That’s like saying the guy who kicks in your door and holds a syringe to your neck isn’t part of the assault, he’s just delivering the threat. The LNP is a pharmacological agent. It modulates the immune system, disrupts membranes, and induces inflammation all on its own, even when it’s carrying nothing. That was proven years ago.
The pharmaceutical companies knew it. The regulators knew it. The journals buried it.
This entire “payload” framing was sleight of hand, it kept your eyes on the mRNA so you wouldn’t ask too many questions about the shell. But the shell is a very big part of the story. Because you can’t outrun a system-wide immune trigger, and you sure as hell can’t undo what it does to kids, brains, hearts, or long-term inflammation cascades.
So again, the question no one in power wants you to ask:
When does the drug begin?
It's a complicated PROCESS! Sigh! Thank you for emphasizing this. To me, it looks like a stochastic assembly and de-assembly -- the body will try to do something with it. And then, taking the parts, disseminating them, and in yet new cellular environments, they will get assembled and taken apart again. I don't know what it would take for this process to be terminated, and the various objects to be finally eliminated from the body. Do you have any indicators that this can happen? To me, it seems that the individual compounds are not something that Mother Nature needs and intends to have. Natural substances are recycled and, in this sense, never lost. No process can ever truly end. Even if one terminates, it affects countless others. So, your work reveals frustrating aspects of this LNP process that most don't appreciate. But it may be that even THAT is merely the tip of the iceberg :-(